Activity & Practical Acid & Base React with Metals| Reaction of zinc granules with dilute sulphuric acid testing of hydrogen gas by burning experiment | Class 10th level | edugrown
When we pour dilute sulphuric acid on zinc granules, zinc sulphate is formed and the hydrogen gas is evolved with an effervescence. On reaction of zinc with sulphuric acid the salt formed is a white colour substance which is zinc sulphate which is also known as white vitriol.

Aim
To carry out the Reaction of Zinc with dilute sulphuric acid and classify it as physical or chemical changes
MATERIALS REQUIRED
Chemicals Required
Zinc Granules, Dilute Sulphuric Acid
Apparatus Required
Big hard Glass Test Tube, Jet Delivery Tube, Stand with Clamp, Cork, Match Stick
THEORY
Physical Change: These changes can be observed and take place without changing the composition of substances. There are no changes in chemical nature of substance. The changes which can be observed are like change in colour, boiling point, melting point, hardness, density, fluidity, rigidity etc. The interconversion of states of matter is also a physical change.
Chemical change: In this change, chemical reaction takes place and completely a new substance with different properties is formed. There are changes in chemical properties and composition of substance.
ex: 2Mg(s) + O2(g) → 2MgO(s)
Here, the chemical composition and properties of product MgO is totally different from its reactants Mg and O.
PROCEDURE
Step 2: Few pieces of zinc is put in the test tube.
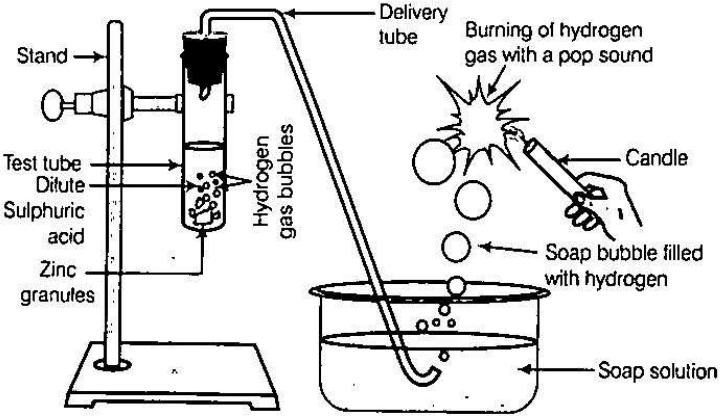
OBSERVATIONS
1. After adding dil. H2SO4 in the tube a brisk reaction starts and gas bubbles come out.
2. On bringing the flame, the gas coming out of the jet burns instantaneously with a small explosion or pop up sound which shows the presence of hydrogen gas.
RESULT
1. The gas produced in the reaction is hydrogen. Zinc reacts with dilute sulphuric acid to produce hydrogen gas (H2) and zinc sulphate.
Zn(s) + H2SO4(g) → ZnSO4(s) + H2
(Zinc) (dil. sulphuric acid) (Zinc Sulphate) (Hydrogen gas)
This is an example of displacement reaction of a non-metal by a metal.
2. Zinc is more reactive than hydrogen.
4.ZnSO4 is different in chemical composition and chemical properties than Zn and H2SO4 so it is a chemical change.
PRECAUTIONS
1. Use the chemicals judiciously.
2. Do not inhale gases evolved directly.
3. Hydrogen gas instantaneously burns with mild explosion. Therefore, a fine jet tube should be used to see the burning of hydrogen.
4. Handle acids and alkalies carefully
Related
Discover more from EduGrown School
Subscribe to get the latest posts sent to your email.
