NCERT Solutions for Class 11 Chemistry Chapter -9 Hydrogen includes all the important topics with detailed explanation that aims to help students to understand the concepts better. Students who are preparing for their Class 11 Chemistry exams must go through NCERT Solutions for Class 11 Chemisrty Chapter -9 Hydrogen. NCERT Solutions will make you understand the topics in most simple manner and grasp it easily to perform better.
NCERT Solutions for Class 11 Chemistry Part 2
There are seven chapters in Class 11 Chemistry Part 2 textbook which will make you well versed in variety of topics and allows students to cover the entire syllabus effectively without any frustration. These NCERT Solutions are curated by the experts in a comprehensive which can be helpful in clearing your doubts instantly.
Class 11th Chapter -9 Hydrogen | NCERT CHEMISRTY SOLUTION |
NCERT Exercises
Question 1.
Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Solution.
Hydrogen has electronic configuration 1s1. Its electronic configuration is similar to the outer electronic configuration (ns1) of alkali metals, which belong to the first group of the periodic table.
Question 2.
Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes?
Solution.
Hydrogen has three isotopes :
The mass ratio of hydrogen isotopes is 1 : 2 : 3.
Question 3.
Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Solution.
In monoatomic form hydrogen atom has only one electron in K-shell (1s1) while in diatomic form, the K-shell is complete (1s2). This means that in diatomic form, hydrogen (H2) has acquired the configuration of nearest noble gas, helium. It is therefore, quite stable.
Question 4.
How can the production of dihydrogen, obtained from ‘coal gasification’, be increased?
Solution.
The process of producing ‘syngas’ from coal is called “coal gasification”.![]()
The production of dihydrogen can be increased by reacting carbon monoxide of syngas mixtures with steam in the presence of iron chromate as catalyst.![]()
This is called water-gas shift reaction.
Question 5.
Describe the bulk preparation of dihydrogen by electrolytic method. What is the role of an electrolyte in this process?
Solution.
Dihydrogen is prepared by the electrolysis of water using platinum electrodes in the presence of a small amount of acid or alkali.![]()
During electrolysis, the reactions that take place are :
Question 6.
Complete the following reactions.
Solution.
Question 7.
Discuss the consequences of high enthalpy of H — H bond in terms of chemical reactivity of dihydrogen.
Solution.
Dihydrogen is quite stable and dissociates into hydrogen atoms only when heated at about 2000 K.![]()
Above 2000 K, the dissociation of hydrogen into atoms is only 0.081% which increases to 95.5% at 5000 K. Its bond dissociation energy is very high.![]()
Due to its high bond dissociation energy it is not very reactive.
Question 8.
What do you understand by
- electron- deficient,
- electron-precise and
- electron- rich compounds of hydrogen?
Provide justification with suitable examples.
Solution.
- Electron-deficient hydrides : The hydrides of group 13 elements have an incomplete octet and hence are electron deficient molecules. They act as Lewis acids e.g., B2H6
- Electron-precise hydrides : These compounds have the required number of electrons to write their conventional Lewis structures. All elements of group 14 form such compounds (e.g., CH4) which have tetrahedral structure.
- Electron rich compounds of hydrogen : These compounds have excess electrons which are present as lone pairs, elements of group 15-17 form such compounds, e.g., NH3 has one lone pair, H2O has two lone pairs and HF has three lone pairs.
Question 9.
What characteristics do you expect from an electron-deficient hydride with respect to its structure and chemical reactions?
Solution.
It is expected to be a Lewis acid. For example, diborane B2H6 (dimer of BH3) forms complex with LiH which gives H– ion (Lewis base).
Question 10.
Do you expect the carbon hydrides of the type (CnH2n + 2) to act as Lewis acid or base? Justify your answer.
Solution.
The type (CnH2n + 2) is not a Lewis acid or a Lewis base because carbon atoms have a complete octet, therefore, hydrides behave as normal covalent hydrides.
Question 11.
What do you understand by the term ‘non-stoichiometric hydrides’? Do you expect this type of the hydrides to be formed by alkali metals? Justify your answer.
Solution.
Hydrides of d-block (Group No. 3, 4, 5 and 6) and f-block are metallic or interstitial hydrides because in these hydrides, hydrogen occupies some interstitial sites in the metal lattice producing distortion. These hydrides are also called non-stoichiometric hydrides as, the law of constant composition does not hold good for them, e.g., LaH2.87, YbH2.55, TiH1.5-1.8, ZrH1.3-1.75, vH0.56, NiH0.6-0.7, PdH0.6-0.8 etc. Alkali metals do not form these types of hydrides because alkali metals donate lone pair of electrons to hydrogen which forms H– ion. As H– ion is formed by complete transfer of electrons so, alkali metal and H atom are in fixed stoichiometric ratio.
Question 12.
How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Solution.
Some metals like palladium (Pd), platinum (Pt) have a tendency to adsorb very large volume of hydrogen and therefore, can be used as its storage media. This property has high potential for hydrogen storage and as a source of energy.
Question 13.
How does the atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes? Explain.
Solution.
Atomic hydrogen torch : When molecular hydrogen is passed through tungsten electric arc ~ 3000°C, at lowr pressure, it dissociates to form atoms of hydrogen known as atomic hydrogen.
When the atoms of hydrogen produced as above are made to fall at some metal surface, they combine to form molecular hydrogen again along with the liberation of large amount of heat energy. The temperature rises to 4000-5000°C. This is the principle of atomic hydrogen torch which is used for welding purposes.
Oxy-hydrogen torch : When hydrogen is burnt in oxygen, the reaction is highly exothermic in nature. A temperature ranging between 2800°C to 4000°C is generated. This temperature can be employed for welding purpose in the form of oxy-hydrogen torch.
Question 14.
Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why?
Solution.
The strength of H-bonding depends upon the magnitude of the polarity of the bond. Since H-F bond has maximum polarity (F is the most electronegative element) hydrogen bonding is maximum in HF molecules.![]()
Question 15.
Saline hydrides are known to react with water violently producing fire. Can CO2, a well-known fire extinguisher, be used in this case? Explain.
Solution.
When saline hydrides react with water, the reaction is highly exothermic, the evolved hydrogen catches fire, e.g.,
Question 16.
Arrange the following :
(i) CaH2, BeH2 and TiH2 in order of increasing electrical conductance.
(ii) LiH, NaH and CsH in order of increasing ionic character.
(iii) H – H, D – D and F – F in order of increasing bond dissociation enthalpy.
(iv) NaH, MgH2 and H2O in order of increasing reducing property.
Solution.
(i) BeH2 is significantly covalent, CaH2 is ionic and TiH2 is metallic hydride. Hence, increasing electrical conductance : BeH2<CaH2<TiH2
(ii) Electronegativity decreases as Li > Na > Cs. Thus, increasing ionic character :
LiH < NaH < CsH.
(iii) Due to lone pairs of F, bond pairs experience repulsion, hence, F-F has low bond dissociation energy. In D-D, due to higher nuclear attraction bond dissociation energy is geater than H- H. Increasing bond dissociation enthalpy : F-F < H-H < D-D.
(iv) NaH is ionic hydride. MgH2 and H2O are covalent hydrides but OH bond in H2O is more stronger. Hence, increasing reducing power :
H2O < MgH2 < NaH.
Question 17.
Compare the structures of H2O and H2O2.
Solution.
In H2O molecule, the oxygen is sp3-hybridised. Two half-filled sp3-orbitals form O – H σ-bonds, while the other two contain lone pairs of electrons. The expected ∠HOH is 109.5°, but the experimental value is 104.5°. This is because lp-lp repulsion > bp-bp repulsion.
On the other hand, H2O2 is a non-planar molecule. The dihedral angle between two planes is 111.5° and ∠OOH is 94.8°. (Gas phase)
Question 18.
What do you understand by the term ‘auto-protolysis’ of water? What is its significance?
Solution.
The reaction in which two water molecules react to give ions with proton transfer is called auto-protolysis of water or k self ionization of water. A proton from one H2O molecule is transferred to another water molecule leaving behind OH– ion and forming a H3O+ ion.
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH–(aq)
Significance : Auto-protolysis proves that water is amphoteric in nature.
Question 19.
Consider the reaction of water with F2 and suggest, in terms of oxidation and reduction, which species are oxidised/reduced?
Solution.
2F2(g) + 2H2O(l) ➝ 4H+(aq) + 4F –(aq) + O2(g)
In this reaction, H2O is oxidised to oxygen by F2. Fluorine has been reduced to F– ion.
Question 20.
Complete the following chemical reactions.
Classify the above into
(a) hydrolysis,
(b) redox and
(c) hydration reactions.
Solution.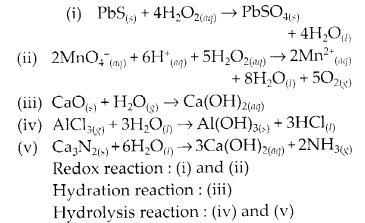
Question 21.
Describe the structure of the common form of ice.
Solution.
Water molecule in solid form is in a perfect tetrahedral shape when each oxygen atom is tetrahedrally (sp3 hybridization) surrounded by four hydrogen atoms, two of which are covalently joint with oxygen and rest of the two form hydrogen bonds with oxygen. This gives ice an open cage-like structure in which each oxygen atom is in contact with four hydrogen atoms and each hydrogen atom is attached to two oxygen atoms (covalently with one and through hydrogen bonding by the other).
Question 22.
What causes temporary and permanent hardness of water?
Solution.
Temporary hardness of water is due to the presence of magnesium and calcium hydrogen carbonates. It can be very easily removed by simply boiling the hard water for sometime. Permanent hardness of water is due to the presence of soluble salts of magnesium and calcium in the form of chlorides and sulphates in water. It cannot be easily removed.
Question 23.
Discuss the principle and method of softening of hard water by synthetic ion-exchange resins.
Solution.
Hard water is made soft by a very common ion exchange resin method. In this the hard water is allowed to pass over a zeolite bed [zeolites are sodium aluminium silicates (Na2Al2Si2O8.xH2O)] during which the Na+ ions from zeolite are replaced by Ca2+ and Mg2+ ions.
Na2Z + Ca2+➝ CaZ + 2Na+
Na2Z + Mg2+ ➝ MgZ + 2Na+
When whole of the Na+ ions of the zeolite have been exchanged, the zeolite is regenerated by treating it with strong (or saturated) solution of NaCl.
MZ + 2NaCl ➝ Na2Z + MCl2 (M = Mg, Ca)
Question 24.
Write chemical reactions to show the amphoteric nature of water.
Solution.
Water is amphoteric in nature because it can behaves both as an acid and base.
Question 25.
Write chemical reactions to justify that
hydrogen peroxide can function as an oxidising as well as reducing agent.
Solution.
H2O2 acts as oxidising and reducing agent in both acidic as well as in basic medium.
Question 26.
What is meant by ‘demineralised’ water and how can it be obtained?
Solution.
Question 27.
Is demineralised or distilled water useful for drinking purposes? If not, how can it be made useful?
Solution.
No, demineralised water is not always useful for drinking purposes. It is usually tasteless. Moreover, some ions such as Na+, K+ and Mg2+, etc. are essential to the body.
In order to make demineralised water more useful, proper amount of additional salts of sodium and potassium etc. must be dissolved in it.
Question 28.
Describe the usefulness of water in biosphere and biological systems.
Solution.
A major part of all living organisms is made up of water. It is a crucial compound for the survival of all forms of life. It is a solvent of great importance.
Liquid water is found in water bodies, such as ocean, sea, lake, river, stream, canal, pond, or puddle. The majority of water on the Earth is ocean water. Water is important in both chemical and physical weathering processes at the Earth’s surface. Water exists as vapours in the atmosphere.
All known forms of life depend on water. Water is vital both as a solvent in which many of the body’s solutes dissolve and as an essential part of many metabolic processes within the body.
Question 29.
What properties of water make it useful as a solvent? What types of compound can it
(i) dissolve, and
(ii) hydrolyse?
Solution.
Water is useful as a solvent rather excellent solvent due to the following properties :
(a) It is a liquid over a wide range of temperature (0° to 100°C).
(b) It has high enthalpv of vapourisation and heat capacitv.
(c) It is polar in nature and has a high dielectric constant (78.39).
(i) It can dissolve polar substances and also some organic compounds due to hydrogen bonding.
(ii) Water can hydrolyse oxides, halides, phosphides, nitrides, etc due to interionic attraction between them.
Question 30.
Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes?
Solution.
No, heavy water (D2O) cannot be used for drinking purposes because it is injurious to health due to presence of D+ ions. Heavy water of high concentration retards the growth of plants and animals. Heavy water has germicide and bactericide properties.
Question 31.
What is the difference between the term ‘hydrolysis’ and ‘hydration’?
Solution.
When H+ and OH– ions of H2O interacts with anion and cation of the salt respectively of give the original acid and base then the reaction is called hydrolysis. It results in the change of pH of solution.
When H2O is added to ion or molecule to give hydrated ion or compound then the reaction is called hydration.
Question 32.
How can saline hydrides remove traces of water from organic compounds?
Solution.
Saline hydrides such as NaH release H– ions which act as strong Bronsted base whereas H2O is a weak Bronsted acid. They combine with water to liberate hydrogen gas.
NaH + H2O ➝NaOH + H2
As a result, these hydrides can be used to remove traces of water from organic compounds.
Question 33.
What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15, 19, 23 and 44 with dihydrogen? Compare their behaviour towards water.
Solution.
The elements with atomic numbers 15, 19, 23 and 44 are phosphorus (P), potassium (K), vanadium (V) and ruthenium (Ru) respectively. The nature of their respective hydrides is :
PH3 (Molecular hydride), KH (Ionic), interstitial or metallic hydrides with vanadium (V). Ruthenium being a transition metal of group 8 does not form hydride. PH3 acts as a weak base with water. KH reacts violenty with water to give KOH and H2.
Question 34.
Do you expect different products in solution when aluminium(III) chloride and potassium chloride are treated separately with
(i) normal water
(ii) acidified water, and
(iii) alkaline water?
Write equations wherever necessary.
Solution.
Both the compounds are salts and they react differently with water.
Aluminium (III) chloride or A1C13 will react with water as follows :
AlCl3 + 3H2O ➝ Al(OH)3 + 3HCl.
The reaction is known as hydrolysis.
(i) In normal water, both Al(OH)3 and HCl will be present.
Question 35.
How does H2O2 behave as a bleaching agent?
Solution.
H2O2 acts as bleaching agent due to the release of nascent oxygen.
H2O2 ➝ H2O + [O]
Thus, the bleaching action of hydrogen peroxide is permanent and is due to oxidation. It oxidises the colouring matter to a colourless product.
Question 36.
What do you understand by the terms:
- hydrogen economy
- hydrogenation
- syngas
- water-gas shift reaction
- fuel-cell ?
Solution.
- Hydrogen economy : The energy is transported and stored in the form of liquid or gaseous hydrogen is the principle due to which hydrogen is considered to be a possible source of clean energy. This proposal to use hydrogen as fuel is called hydrogen economy.
- Hydrogenation : The process of addition of hydrogen to unsaturated hydrocarbons is known as hydrogenation.

- Syngas : The mixture of CO and H2 is used for the synthesis of methanol and a number of hydrocarbons, it is also called synthesis gas or syngas. Nowadays, syngas is produced from sewage, saw dust, scrap wood, newspapers, etc.
- Water-gas shift reaction: The production of dihydrogen can be increased by reacting CO of syngas mixtures with steam in the presence of iron chromate as catalyst.

This is called water-gas shift reaction. - Fuel cell: It is a commercial cell in which the chemical energy produced during the combustion of fuel is converted directly into electricity, e.g., H2 – O2 fuel cell. A fuel cell is used as a source of electrical energy in the space vehicles.
Related
Discover more from EduGrown School
Subscribe to get the latest posts sent to your email.
