The s-Block Elements Class 11 Notes Chemistry
The s-Block Elements
The s-block elements of the Periodic Table are those in which the last electron enters the outermost s-orbital. As the s-orbital can accommodate only two electrons, two groups (1 & 2) belong to the s-block of the Periodic Table.
Group-1 of periodic table contains : Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs) and Francium (Fr). Together these elements are called alkali metals because they form hydroxides on reaction with water, which are strongly alkaline in nature.
The group-2 includes Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). Except Beryllium, rest of the elements of group-2 are called the alkaline earth metals. These are called so because their oxides and hydroxides are alkaline in nature and these metal oxides are found in the earth crust.
Group-1 Elements : Alkali Metals
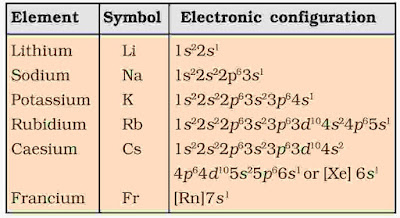
1. Electronic Configuration
Electronic Configuration of elements of group-1 is ns1, where n represents the valence shell. The alkali metals have one valence electron, outside the noble gas core.
2. Atomic and ionic radii
The atoms of alkali metals have the largest size in their respective periods. The atomic radius increases on moving down the group because on moving down the group there is a progressive addition of new energy shells.
3. Ionization enthalpy
The ionization enthalpies of the alkali metals are generally low and decrease down the group from Li to Cs. This is because on moving down the group is due to increase in size of the atoms of alkali metals and increase in the magnitude of screening effect.
4. Hydration enthalpy
The alkali metal ions are extensively hydrated in aqueous solutions. The hydration enthalpies of alkali metal ions decrease with increase in ionic size Li+ > Na+ > K+ > Rb+ > Cs+.
5. Physical properties
- Alkali metals are silvery white in colour and are generally soft and light metals.
- The densities of alkali metals are low and increase down the group.Alkali metals have low melting and boiling point.
- When alkali are heated metals they impart characteristic colours to the flame.
- When the excited electron comes back to the ground state, there is emission of radiation in the visible region.
6. Chemical Properties
The alkali metals are highly reactive elements. The cause for their high chemical reactivity is
- (i) Low value of first ionisation enthalpy
- (ii) Large size
- (iii) low heat of atomisation.
(i). Reaction with Air: Alkali metals burn very fast in oxygen and form different kind of oxides like monoxides, peroxides and superoxides. In all the compounds formed by alkali metals with oxygen, their oxidation state is +1.
4Li + O2 ⟶ 2Li2O (Oxide)
Na + O2 ⟶ Na2O2 (Peroxide)
M + O2 ⟶ MO2 (Superoxide)
(ii). Reaction with Water: The alkali metals on reaction with water form their respective hydroxide and dihydrogen.
2M + 2H2O ⟶ 2M+ + 2OH– + H2
(M = an alkali metal)
(iii). Reaction with Dihydrogen: Alkali metal react with dry di-hydrogen at about 673 K (lithium at 1073 K) to form crystalline hydrides which are ionic in nature and have high melting points.
2M + H2 ⟶ 2M+H–
(iv). Reaction with Halogens: The alkali metals react vigorously with halogens and form halides which are ionic in nature, M+X–. But the halides of lithium are a bit covalent in nature.
(v). Reaction with Mercury: The alkali metals have strong tendency to get oxidised, that is why they act as strong reducing agents, among these lithium is the strongest and sodium is the least powerful reducing agent.
(vi). Reducing Nature: Alkali metals combine with mercury to form amalgams. The reaction is highly exothermic in nature.
Na + Hg ⟶ Na[Hg]
(vii). Solutions in liquid Ammonia: All alkali metals dissolve in liquid ammonia and give deep blue colour solution which are conducting in nature. These solutions contain ammoniated cations and ammoniated electrons as shown below:
M + (x + y)NH3 ⟶ [M(NH3)x]+ + [e(NH3)y]–
Uses of Alkali Metals
- Lithium is used as a metal in a number of alloys. Its alloys with aluminium to make aircraft parts.
- Lithium hydroxides is used in the ventilation systems of space crafts and submarines to absorb carbondioxide.
- Lithium aluminium hydride (LiAlH4) is a powerful reducing agent which is commonly used in organic synthesis.
- Liquid sodium or its alloys with potassium is used as a coolant in nuclear reactors.
- Sodium-lead alloy is used for the preparation of tetraethyl lead, Pb(C2H5)4, which is used as an antiknocking agent in petrol.
- Sodium is used in the production of sodium vapour lamps.
- Potassium chloride is used as fertilizer.
- Potassium hydroxide is used in the manufacture of soft soaps and also as absorbent of carbon dioxide.
- Potassium ions play a vital role in biological systems.
- Caesium is used in photoelectric cells.
Anomalous Properties of Lithium
Lithium shows properties which are very different from the other members of its group. This is due to the
- exceptionally small size of its atom and ion.
- greater polarizing power of lithium ion.
- as compared to other alkali metals, lithium is harder and its melting point and boiling point are higher.
- among all the alkali metals lithium is least reactive but the strongest reducing agent.
Some important Compounds of Sodium
Sodium is highly reactive and always found in combined state. The isotope of sodium (Na) is used in detection of leukemia. The compound of sodium are given below:
1. Sodium Oxide (Na2O)
Preparation:
2NaNO2 + 6Na ⟶ 4Na2O + N2
Properties:
- Sodium oxide is a colourless ionic solid.
- Aqueous solution of sodium oxide is strongly basic.
- Sodium oxide on reaction with liquid ammonia forms sodamide.
- At low temperature, when sodium peroxide is reacted with water or acids, H2O2 is formed.
2. Sodium Peroxide (Na2O2)
Preparation:
Sodium when heated in excess of air or when heated in excess of pure oxygen gives sodium peroxide.
2Na + O2 ⟶ Na2O2
Properties:
- Sodium peroxide is a pale yellow diamagnetic compound.
- Sodium peroxide is a powerful oxidising agent.
- Sodium peroxide combines with CO and CO2 to give carbonate.
- At low temperature, when sodium peroxide is reacted with water or acids, H2O2 is formed.
Na2O2 + 2H2O ⟶ 2NaOH + H2O2
3. Sodium Hydroxide (Caustic Soda) (NaOH)
Preparation:
When sodium carbonate is treated with calcium hydroxide it give calcium carbonate along with sodium hydroxide. Also known as lime caustic soda process. It is a reversible reaction.
Na2CO3 + Ca(OH)2 ⟶ 2NaOH + CaCO3
Properties
- Sodium hydroxide is a white crystalline deliquescent solid.
- Sodium hydroxide is corrosive in nature.
- Sodium hydroxide is highly soluble in water.
- Sodium hydroxide reacts with acid forming corresponding salts.
NaOH + HCl ⟶ NaCl + H2O
Uses: It is used in
the manufacture of soap, paper, artificial silk and a number of chemicals,
- in petroleum refining,
- in the purification of bauxite,
- in the textile industries for mercerising cotton fabrics,
- for the preparation of pure fats and oils, and
- as a laboratory reagent.
4. Sodium Carbonate (Na2CO3)
Preparation:
NH3 + H2O + CO2 ⟶ NH4HCO3
NaCl + NH4HCO3 ⟶ NaHCO3 + NH4Cl
2NaHCO3 ⟶ Na2CO3 + H2O + CO2
Properties:
- Sodium carbonate is a white crystalline solid.
- Na2CO3.10H2O is known as washing soda.
- Sodium carbonate reacts with acids to give carbon dioxide.
Uses:
- It is used in water softening, laundering and cleaning.
- It is used in the manufacture of glass, soap, borax and caustic soda.
- It is used in paper, paints and textile industries.
- It is an important laboratory reagent both in qualitative and quantitative analysis.
Na2CO3 + HCl ⟶ NaCl + H2O + CO2
Properties:
On heating sodium bicarbonate loses CO2 and H2O forming Na2CO3.
2NaHCO3 ⟶ Na2CO3 + H2O + CO2
6. Sodium Chloride (NaCl)
Manufacture of sodium chloride is done from sea water. Sea water is allowed to dry up under summer heat in small tanks and solid crust so formed is collected.
Properties:
- Sodium chloride is a white crystalline solid.
- It is slightly hygroscopic.
- It is soluble in water and insoluble in alcohol.
Uses:
- It is used as a common salt or table salt for domestic purpose.
- It is used for the preparation of Na2O2, NaOH and Na2CO3.
5. Sodium Bicarbonate (Baking Soda) (NaHCO3)
Preparation:
When NaOH is treated with CO2 in presence of H2O it gives sodium bicarbonate.
NaOH + CO2 + H2O ⟶ NaHCO3
Properties:
On heating sodium bicarbonate loses CO2 and H2O forming Na2CO3.
2NaHCO3 ⟶ Na2CO3 + H2O + CO2
Group-2 Elements : Alkaline Earth Metals
The elements of group-2 are Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). Except for Be, rest are known as alkaline earth metals, because they were alkaline in nature and existed in the earth.
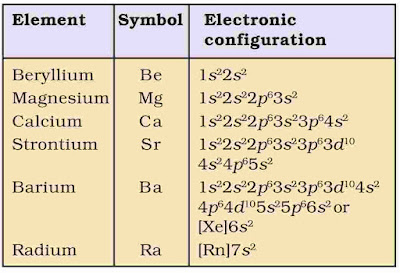
1. Electronic Configuration
The alkaline earth metals have 2 electrons in the s-orbital of the valence shell. Their general electroni configuration [Noble gas]ns2
2. Atomic and Ionic Radii
The atomic radii as well as ionic radii of the members of the family are smaller than the corresponding members of alkali metals. Within the group, the atomic and ionic radii increase with increase in atomic number.
3. Ionization Enthalpies
The alkaline earth metals owing to their large size of atoms have fairly low values of ionization enthalpies. Within the group, the ionization enthalpy decreases as the atomic number increases.
4. Hydration Enthalpies
The hydration enthalpies of alkaline earth metal ions are larger than those of alkali metal ions. Therefore, compounds of alkaline earth metals are more extensively hydrated, for example, magnesium chloride and calcium chloride exist. the hydration enthalpies of alkaline earth metal ions decrease with increase in ionic size down the group. Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
5. Physical Properties
The alkaline earth metals are silvery white, lustrous and relatively soft but harder than the alkali metals.The melting and boiling points of these metals are higher than the corresponding alkali metals.The electropositive character increases down the group from Be to Ba.Calcium, strontium and barium impart characteristic brick red, crimson and apple green colours respectively to the flame.Th alkaline earth metals just like those of alkali metals have high electrical and thermal conductivities.
6. Chemical Properties
As compared to alkali metals, alkaline earth metals are less reactive due to their relatively higher ionization enthalpies. The reactivity of alkaline earth metals increases on going down the group.
(i). Reaction with water: Ca Sr, and Ba have reduction potentials similar to those of corresponding group Ist metals and are quite high in the electrochemical series. They react with cold water readily, liberating hydrogen forming metal hydroxides.
Ca + 2H2O ⟶ Ca(OH)2 + H2
(ii). Reaction with Air: Except Be these metals are easily tarnished in air as a layer of oxide is formed on their surface. Ba in powdered form bursts into flame on exposure to air.
(iii). Reaction with hydrogen: The elements Mg, Ca, Sr and Ba all react with hydrogen to form hydrides MH2.
(iv). Reaction with oxygen: Except Ba and Ra the elements when burnt in oxygen form oxides of the type MO.
(v). Reaction with halogens: When heated with halogens the alkaline earth metals directly combine with them and form the halides of the type MX2.
Ca + Cl2 ⟶ CaCl2
(vi). Reaction with acids: The alkaline earth metals readily react with acids liberating dihydrogen.
M + 2HCl ⟶ MCl2 + H2
Uses of alkaline earth metals
- Beryllium is used in the manufacture of alloys. Cooper-Beryllium alloys are used in the making of high strength springs.
- Metallic beryllium is used for making windows of X-rays tubes.
- Magnesium, being a light metal, forms many light alloys with aluminum, zinc, manganese and tin.
- Magnesium is used in flash powders and bulbs, incendiary bombs and signals.
- Magnesium-aluminium alloys are used in aircraft construction.
- Magnesium is used as sacrificial anode for the prevention of corrosion of iron.
- A suspension of magnesium hydroxide in water (called milk of magnesia) is used as an ant-acid to control excess acidity in stomach.
- Magnesium carbonate is an ingredient of tooth-paste.
- Calcium is used in the extraction of metals from oxides which are difficult to reduce with carbon.
- Calcium and barium metals are used to remove air from vacuum tubes, due to their tendency to react with oxygen and nitrogen at high temperature.
- Radium salts are used for radio therapy of cancer.
Anomalous Behaviour of Beryllium
Beryllium shows different behaviour from the rest members of its group and shows diagonal relationship to aluminium due to reasons discussed below.
- Beryllium has exceptionally small atomic and ionic sizes and therefore does not compare well with other members of the group, because of high ionisation enthalpy and small size it forms compounds which are largely covalent and get easily hydrolysed.
- Beryllium does not exhibit coordination number more than four as in its valence shell, there are only four orbitals. The remaining members of the group can have a coordination number of six by making use of d-orbitals.
- The oxides and hydroxide of beryllium unlike the hydroxide of other elements in the group, are amphoteric in nature.
Compounds of Calcium
1. Calcium Oxide (CaO)
Preparation:
Calcium carbonate when decomposed at 800°C gives calcium oxide.
CaCO3 ⟶ CaO + CO2
Properties:
- Calcium oxide is also known as ‘Quick lime’ or ‘Burnt lime’, is white amorphous substance.
- When water is added to lime a hissing sound is produced along with clouds of steam. The lime forms slaked lime [Ca(OH)2].
- Calcium oxide reacts with moist chlorine to form bleaching powder. CaO + Cl2 ⟶ CaOCl2
- Calcium oxide on reaction with moist HCl gas forms CaCl2.CaO + 2HCl ⟶ CaCl2 + H2O
2. Calcium Carbonate (CaCO3)
Preparation:
Carbon dioxide when passed through lime water gives calcium carbonate.
Ca(OH)2 + CO2 ⟶ CaCO3 + H2O
Properties:
- Calcium carbonate is a white powder insoluble in water.
- Calcium carbonate dissolves in water in presence of CO2 due to formation of calcium bicarbonate.
CaCO3 + H2O + CO2 ⟶ Ca(HCO3)2
3. Calcium Chloride (CaCl2)
Preparation:
Calcium oxide, calcium hydroxide or calcium carbonate when treated with HCl gives calcium chloride.
CaO + 2HCl ⟶ CaCl2 + H2O
Properties:
- Calcium chloride is a colourless deliquescent crystalline substance which is soluble in water as well as in alcohol.
- Crystals of calcium chloride when strongly heated gives off water of crystalisation.
4. Calcium sulphate (Plaster of Paris)
Preparation:
When Gypsum is heated at about 120° – 130°C, Plaster of Paris is formed.
2CaSO4 + 4H2O ⟶ (CaSO4)2H2O + 3H2O
Properties:
- It is a white crystalline solid. It is sparingly soluble in water.
- It becomes anhydrous at about 200°C. Anhydrous form is known as dead burnt plaster.
5. Calcium hydroxide Ca(OH)2
Preparation:
CaO + H2O ⟶ Ca(OH)2
Properties:
1. It gives CaCO3 and Ca(HCO3)2 with CO2
Ca(OH)2 + CO2 ⟶ CaCO3 + H2O
2. On prolong treatment with CO2 milkiness disappears due to formation of Ca(HCO3)2
CaCO3 + H2O + CO2 ⟶ Ca(HCO3)2
Summary
- s-block elements : The elements in which last electron enters into s-orbital are called s-block elements.
- Alkali metals : The elements of group 1 whose hydroxide are strong alkali.
- Alkaline earth metal : The elements of group 2, and their oxides and hydroxides are alkaline in nature and their oxides are found in the Earth’s crust.
- Diagonal relationship : The resemblance in properties of elements of second period with elements of third period present diagonally on the right hand side.
- Monovalent sodium and potassium ions and divalent magnesium and calcium ions are found in large proportions in biological fluids. These ions perform important biological functions such as maintenance of ion balance and nerve impulse conduction.
Discover more from EduGrown School
Subscribe to get the latest posts sent to your email.
