Hydrogen Class 11 Notes Chemistry
Introduction
In this chapter we will study the preparation, properties of dihydrogen and of some important compounds formed by hydrogen like H2O and H2O2.
Hydrogen is the first element of the periodic table. The atomic structure of hydrogen is the most simplest one with only one proton and one electron. Hydrogen occurs in its atomic form only at very high temperatures. Water is one of the most important compounds formed by hydrogen. Even its name hydrogen was given by Lavoisier because of its ability to form water as in Greek, hydro means water and gene means forming.
Position of Hydrogen in the Periodic Table
Hydrogen is the first element in the periodic table. The electronic configuration of hydrogen is 1s1, yet its position in the periodic table is not certain and unsatisfactory. Hydrogen exhibits properties similar to both alkali metals (Group 1) and halogens (Group 17).
Resemblance with Alkali Metals
- Like alkali metals, hydrogen has only one electron in its outer shell.
- Alkali metals have a strong tendency to lose one electron from their outermost shell. Similarly, hydrogen also loses electron to form H+ ion.
- Alkali metals form stable oxides, halides and sulphides. Similarly, hydrogen also forms stable oxide (H2O), halides (HF) and sulphide (H2S).
Resemblance with Halogens
- Halogens have a tendency to gain one electron. Similarly, hydrogen (1s1) gains one electron to form H– ion.
- Hydrogen molecule is diatomic (H2) and so are the molecules of halogens (say F2).
- Hydrogen forms hydrides with carbon (e.g., CH4), just like halogens form halides with carbon (CCl4).
Isotopes of Hydrogen
Isotopes are the different forms of the same element having same atomic number but different mass numbers. There are three isotopes of hydrogen namely protium, deuterium and tritium.
1. Protium or ordinary hydrogen (1H1): It has one proton and no neutron in the nucleus and one electron revolves around the nucleus.
2. Deuterium (1H2 or D): It is also known as heavy hydrogen. It has one proton and one neutron in the nucleus around which one electron revolves.
3. Tritium (1H3 or T): This isotope of hydrogen is radioactive and emits low energy β– particles having half life period of 12.33 years. It has one proton and two neutrons in the nucleus. The concentration of tritium is very low.
Dihydrogen
Occurrence
Dihydrogen is the most abundant element in the universe. It constitutes about 70% of the total mass of the universe. But its abundance in earth’s atmosphere is very less. It is just 0.15% by mass in the earth’s atmosphere. In free state hydrogen is present in volcanic gases and in the combined form it constitutes 15.4% of the earth’s crust and the oceans. However, it is also present in the plant and animal tissues, carbohydrates, proteins etc. Even hydrogen is present in mineral resources like coal and petroleum.
Hydrogen is the principal element in the solar atmosphere. It is present in the outer atmosphere of Sun and other stars of the universe like Jupiter and Saturn.
Preparation of Dihydrogen
1. Laboratory Preparation of Dihydrogen
(i) In laboratory dihydrogen is prepared by the reaction of granulated zinc with dilute hydrochloric acid or dilute sulphuric acid.
Zn + 2H+(dil) ⟶ Zn2+ + H2
(ii) Zinc reacts with aqueous alkali to give dihydrogen
Zn + 2NaOH ⟶ Na2ZnO + H2
2. Commercial Production of Dihydrogen
(i). By the electrolysis of water : Electrolysis of acidified water using platinum electrodes is used for the bulk preparation of hydrogen.
2H2O ⟶ 2H2 + O2
(ii). By the action of steam on coke : Dihydrogen is prepared by passing steam over coke or hydrocarbons at high temperature (1270 K) in the presence of Nickel catalyst.
C + H2O ⟶ CO + H2
The mixture of CO(g) and H2(g) is called water gas. It is also known as synthesis gas or simply ‘syn gas’ because it is used in the synthesis of methanol and many other hydrocarbons.
Properties of Dihydrogen
(i). Physical Properties
- Dihydrogen is a colourless, odourless, tasteless, combustible gas.
- It is lighter than air.
- It is insoluble in water.
(ii). Chemical Properties
Reaction with halogens: It reacts with halogens, X2 to give hydrogen halides, HX,
H2 + X2 ⟶ 2HX (X F,Cl, Br,I)
Reaction with dioxygen: It reacts with dioxygen to form water. The reaction is highly exothermic.
2H2 + O2 ⟶ 2H2O
Reaction with dinitrogen: With dinitrogen it forms ammonia.
3H2 + N2 ⟶ NH3
Reactions with metals: Dihydrogen reacts with metals to yield hydrides at high temperature.
H2 + 2M(g) ⟶ 2MH(s)
where M is an alkali metal.
Hydrogenation of vegetable oils: Edible oils (unsaturated) like cotton seed oil, groundnut oil are converted into solid fat (saturated) also called vegetable ghee by passing hydrogen through it in the presence of Ni at 473 K.
Vegetable oil + H2 ⟶ Fat
Uses of Dihydrogen
1. Synthesis of ammonia: Dihydrogen is used in Haber’s process in the synthesis of ammonia.
2. Hydrogenation of oils: Dihydrogen is added to oils like soyabean oil, cotton seed oil for manufacturing vanaspati fat.
3. Manufacture of methyl alcohol: Water gas enriched with hydrogen gas in the presence of cobalt catalyst gives methanol.
4. Manufacture of hydrogen chloride: It is used in the manufacturing of hydrogen chloride which is a very important chemical.
5. Manufacture of metal hydrides: It is used in the manufacture of many metal hydrides.
6. Metallurgical processes: Since, dihydrogen is used to reduce heavy metal oxides to metals, as it is a reducing agent. Therefore, it finds its use in metallurgical processes.
7. Rocket fuel: It is used as a rocket fuel for space research in the form of liquid hydrogen and liquid oxygen.
8. Fuel Cells: Dihydrogen is used in fuel cells for the generation of electrical energy.
9. It is used in the atomic hydrogen torch and oxyhydrogen torches for cutting and welding purposes.
Hydrides
Hydrogen combines with a large number of other elements including metals and non-metals, except noble gases to form binary compounds called hydrides. If ‘E’ is the symbol of the element then hydrides are represented as EHx(e.g., BeH2)
Based on their physical and chemical properties, the hydrides have been classified into three main categories:
- Ionic or saline or salt like hydrides
- Covalent or molecular hydrides
- Metallic or non-stoichiometric hydrides
Ionic or Saline Hydrides
The ionic hydrides are stoichiometric which are formed when hydrogen combines with elements of s-block elements except Be. Ionic hydrides are formed by transfer of electrons from metals to hydrogen atoms and contain hydrogen as H– ion e.g., sodium hydride (Na+H–)
Covalent or Molecular Hydrides
Covalent or molecular hydrides are the compounds of hydrogen with p-block elements. The most common hydrides are CH4, H2O, NH3 etc. Covalent hydrides are volatile compounds.
Metallic or Non-Stoichiometric Hydrides
The elements of group 3, 4, 5 (d-block) and f-block elements form metallic hydrides. In group 6, only chromium forms hydride (CrH). Metals of group 7, 8, 9 do not form hydrides. These hydrides are known as metallic hydrides because they conduct electricity.
Water
Water is one of the most readily available chemical. Water is an oxide of hydrogen. It is an important component of all living organisms. Water constitutes about 65% of human body and 95% of plants. It is therefore essential for life. The ability of water to dissolve so many other substances makes it a compound of great importance. Almost three-fourth of the earth’s surface is covered with water.
Physical Properties of Water
- Pure water is colourless, odourless and tasteless.
- Water is present in the liquid state at room temperature.
- Water boils at 100°C and changes into the gaseous state whereas it freezes at 0°C to form ice.
- Water molecules undergo extensive hydrogen bonding.
- It is an excellent solvent for many thing like alcohols and carbohydrates dissolve in water.
Structure of Water

Structure of Ice
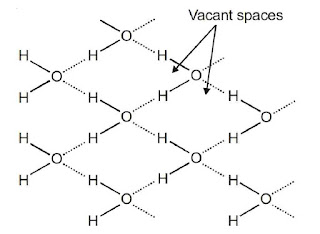
Chemical Properties
1. Amphoteric nature: Water can act both as an acid as well as a base and is thus said to be an amphoteric substance.
Water as base: Water acts as a base towards acids stronger than it as shown below,
H2O + HCl ⟶ H3O+ + Cl–
Water as an acid: Water acts as an acid towards bases stronger than it
H2O + NH3 ⟶ OH– + NH4+
2. Redox reactions involving water: Water can act both as oxidising as well as reducing agent.
Oxidising agent: Water acts as an oxidising agent when it gets reduced.
2H2O + 2Na ⟶ 2NaOH + H2
Reducing agent: Water acts as a reducing agent when it gets oxidised.
2H2O + 2F2 ⟶ 4H+ + 4F– + O2
3. Hydrolysis reaction: Water is an excellent solvent due to its high dielectric constant (78.39). In addition, water can easily hydrolyse many ionic and covalent compounds.
(i) Water hydrolyses oxides and halides of non-metals forming their respective acids
P4O10 + 6H2O ⟶ 4H3PO4
4. Hydrates Formation: From aqueous solutions many salts can be crystallised as hydrated salts. Hydrates are of three types:
(i) Coordinated water
For example: [Ni(H2O)6]2+ (NO3–)2 and [Fe(H2O)6]Cl3
(ii) Interstitial water
For example: BaCl2.2H2O
(iii) Hydrogen bonded water
For example: [Cu(H2O)4]2+ SO42- H20 in CuS04.5H2O
Hard and Soft Water
Hard water is the one which does not produce lather with soap easily due to the presence of calcium and magnesium salts in the form of their bicarbonates, chlorides and sulphates. For example, sea water etc.
Soft water is the one which is free from the soluble salts of calcium and magnesium. It gives lather with soap easily. For example, distilled water, rain water etc.
Types of Hardness
1. Temporary hardness: It is due to the presence of bicarbonates of calcium and magnesium. Temporary hardness is called so because it can be easily removed by boiling.
2. Permanent hardness: This type of hardness is due to the presence of chlorides and sulphates of calcium and magnesium dissolved in water. As this type of hardness cannot be removed by simple boiling, therefore it is known as permanent hardness.
Softening of Water
The process of removal of hardness from water is called softening of water.
(i) Removal of temporary hardness: Temporary hardness can be removed by the following methods:
(a) Boiling: The temporary hardness of water can easily be removed by boiling the water in large boilers. During boiling the soluble Mg(HCO3)2 is converted into Mg(OH)2 instead of MgCO3 because Mg(OH)2 is precipitated easily, whereas Ca(HCO3)2 is changed to insoluble CaCO3 and gets precipitated. These precipitates can be removed by filtration process. So, the filtrate obtained will be soft water.
Mg(HCO3)2 ⟶ Mg(OH)2 + 2CO2
Ca(HCO3)2 ⟶ Ca(OH)2 + H2O + 2CO2
(b) Clark’s method: In this process the calculated amount of lime is added to hard water containing bicarbonates of calcium and magnesium. It precipitates out calcium carbonate and magnesium hydroxide which are then filtered to obtain soft water.
Ca(HCO3)2 + Ca(OH)2 ⟶ 2CaCO3↓ + 2H2O
Mg(HCO3)2 + 2Ca(OH)2 ⟶ 2CaCO3↓ + Mg(OH)2↓ + 2H2O
(ii) Permanent hardness: Permanent hardness of water is due to the presence of chlorides and sulphates of calcium and magnesium. It cannot be removed by simple boiling. So, the following methods are employed for removing permanent hardness:
(a) Treatment with washing soda: When calculated amount of Na2CO3 (washing soda) is added to hard water containing soluble sulphates and chlorides of calcium and magnesium, then these soluble salts get converted into insoluble carbonates which get precipitated.
CaCl2 + Na2CO3 ⟶ 3CaCO3↓ + 2NaCl
MgSO4 + Na2CO3 ⟶ 3MgCO3↓ + Na2SO4
(b) Ion-exchange method: This process employs the use of zeolite or permutit which is hydrated sodium aluminium silicate (NaAlSiO4), therefore, it is also known as zeolite/permutit process. For the sake of simplicity sodium aluminium silicate is written as NaZ. When zeolite is added to hard water, the cations present in hard water are exchanged for sodium ions.
2NaZ(s) + M2+(aq) ⟶ MZ2(s) + 2Na+(aq) (M = Mg, Ca)
Hydrogen Peroxide
Hydrogen peroxide was discovered by a French chemist J. L. Thenard. It is an important chemical used in pollution control treatment of domestic and industrial effluents.
Preparation
By the action of sulphuric acid on hydrated barium peroxide
BaO.8H2O + H2SO4 ⟶ BaSO4 + H2O2 + H2O
Physical Properties
- Pure hydrogen peroxide is a syrupy liquid. It is colourless but gives a bluish tinge in thick layers.
- It is soluble in water, alcohol and ether in all proportions.
- It is more viscous than water. This is due to the fact that molecules of H2O2 are more associated through H-bonding.
Structure
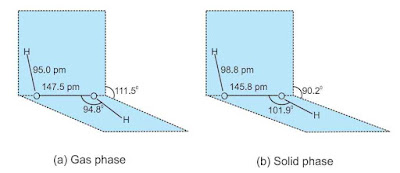
Chemical Properties
(a) Oxidising property: Hydrogen peroxide acts as an oxidising agent both in acidic as well as in alkaline medium.
H2O2 + 2H+ + 2e– ⟶ 2H2O
(b) Reducing Property: In presence of strong oxidising agents, hydrogen peroxide behaves as a reducing agent in both the medium.
H2O2 + O3 ⟶ H2O + 2O2
(c) Decomposition: H2O2 is an unstable liquid
2H2O2 ⟶ 2H2O + O2
Uses
- In daily life it is used as a material to bleach delicate materials like hair, cotton, wool, silk etc.
- It is used as a mild disinfectant. It is also a valuable antiseptic which is sold under the name of perhydrol.
- In the manufacture of sodium perborate, sodium percarbonate. These are used in high quality detergents.
- In the synthesis of hydroquinone, tartaric acid and certain food products and pharmaceuticals (cephalosporin) etc.
- It is used in industries as a bleaching agent for paper pulp, leather, oils, fats and textiles etc.
Heavy Water (D2O)
Heavy water is chemically deuterium oxide (D2O). It was discovered by Urey in 1932.
Preparation
It is prepared by the exhaustive electrolysis of water. When prolonged electrolysis of water is done, then H2 is liberated much faster than D2 and the remaining water becomes enriched in heavy water.
H2O + D2 ⟶ D2O + H2
Uses
- Heavy water is used as a moderator in nuclear reactors.
- It is used as a tracer compound, in studying the reaction mechanisms.
- It is used as a starting material for the preparation of a number of deuterium compounds.
Discover more from EduGrown School
Subscribe to get the latest posts sent to your email.
