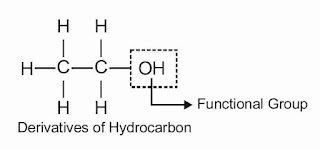
Chapter 1- The Living World | class 11th | revision notes biology
The Living World Class 11 Notes Biology Chapter 1
1. Living World: Characteristics
Non-living things like mountains, boulders, sand dunes also grow in size, but just by accumulating the material on their external surface. Thus, growth in living things is internal, while in non-living things, it is external. It is to be noted that a dead organism do not grow.
2. Reproduction
Reproduction, a characteristic of living organisms is the process of producing offsprings, possessing features similar to those of parents. In multicellular organisms, the mode of reproduction is generally sexual. Living organisms also reproduce by asexual means.
Some examples are given below
(i) Fungi spread and multiply fast by producing millions of asexual spores. Some fungi, the filamentous algae and the protonema of mosses multiply by fragmentation.
(ii) In yeast and Hydra, budding occurs to produce new organisms. While, in Planaria (flatworm),
regeneration of fragmented body parts occur. These parts inturn grow as a new organism.
(iii) Unicellular organisms like bacteria, algae and Amoeba reproduce by increasing the number of cells, i.e., through cell division (growth is synonymous with reproduction).
Some organisms like mules, sterile worker bees, infertile human couples, etc., do not reproduce. Hence, reproduction also cannot be an all-inclusive defining characteristic of living organisms.

3. Metabolism
Metabolism is an another characteristic and defining feature of all living things. The sum total of anabolic or constructive reactions (anabolism) and catabolic or destructive reactions (catabolism) continuously occurring inside the body is called metabolism.
Metabolism —> Anabolism + Catabolism Metabolism occurs in all unicellular and multi cellular organisms. Its two stages include, i.e., anabolism, the process of building up or synthesis of complex substances from simpler ones, e.g., Photo synthesis and catabolism, the process of breakdown of complex substances into simpler substances, e.g., Respiration, releasing waste outside.
Metabolic reactions can also be demonstrated outside the body in cell free systems, which are neither living nor non-living. Thus, these reactions in vitro are surely living reactions not living things. Hence, metabolism can be considered as a defining feature of all living organisms without exception.
The important differences between anabolism and catabolism are
Viruses are considered as non-living because they don’t need energy for their activities, i.e., metabolic activities are altogether absent in them.
4. Cellular Organisation
The cells are the building blocks of all living things whether plants, animals or humans. The unicellular organisms are made of a single cell, while multi cellular organisms are formed by millions of cells. The cells contain protoplasm (living matter) and cell organelles (inside the cells) which perform several activities at the cellular level and result into various life processes.
5. Consciousness
All living organisms have excellent ability to sense their environment. They respond to various physical, chemical and biological stimuli.
The various external factors to which living organisms respond are light, water, temperature, pollutants, other organisms, etc. Light duration or photo period affects many seasonal breeders, plants as well as animals. All living things respond to chemicals, entering their * bodies.
Humans are superior to all living things as they have an additional ability of self-consciousness. Therefore, consciousness can also said to be a defining property of living organisms.
However, in human beings, it is more difficult to define living state, e.g., Patients lying in coma supported by machines that replace heart and lungs, are brain-dead with no self-consciousness.
6. Body Organisation
The body of living organisms is organised, i.e., several component and sub-components cooperate with each other for the functioning of whole body.
Physical and Biological Hierarchies
There is a physical (non-living) hierarchy and biological hierarchy in the organisation of living body. In physical hierarchy, various non-living components aggregate to form compounds, which finally enter the living world in the form of cells. These cells organise to form tissues, that form organs and several organs combustive to form organ-systems. Finally, many organ systems organise and form a living organism.
The properties of tissues are not present in the constituent cells but arise as a result of interactions among the constituent cells. For example, bone is a hard tissue, which provides framework to the body. But, the cells present inside it do not have this property. This phenomenon of interactions between various components of the body results in the hierarchy of organisation.
The various life processes are the result of this interaction and coordination. The complexity in organisation enable living organisms as to be self-replicating, evolving, self-regulating and responding to external stimuli. All living organisms along with their ancestors and descendants are linked to one another by sharing of common genetic material in the form of DNA in varying degrees. This DNA is responsible for the expression of specific traits in organisms. Thus, Biology is the story of life on earth. It is the story of evolution of living organisms on the earth.
Some Other Characteristics of Living Organisms
We have discussed some important and defining characteristics of living things. However, organisms . also have many other features that differentiate them from non-living things, such as, shape & size, life cycle, movement, self-regulation, variations, adaptations, healing & repair, excretion and death.
2. Living World : Diversity and Taxonomy
The earth hosts an immense variety of living organisms. According to a survey, the number of species that are known and described are between 1.7-1.8 million.
This number refers to the biodiversity on the earth. The term Biodiversity or Biological diversity means the number and types of organisms present on the earth, forms of life in the living world. The living world includes all the living organisms, such as microorganisms, plants, animals and humans.
Biodiversity is not limited to the existing life forms. If we explore new areas and even old ones, new organisms are continuously being added. This huge available variety cannot be studied and identified without having a proper system of classification and nomenclature.
Systematics
The word ‘Systematics’ is derived from the Latin word Systema, which means systematic arrangement of organisms. Linnaeus used Systema Naturae as the title of his book. He. coined the term Systematics in 1751.
Systematics is the branch of science that deals with unique properties of species and groups to recognise, describe, name and arrange the diverse organisms according to an organised plan.
In 1961, Simpson, defined systematics as the study of diversity of organisms and all their comparative and evolutionary relationships based on comparative’ anatomy, physiology, biochemistry and ecology. The word ‘Systematics’ and ‘Taxonomy’ are often used interchangeably by the biologists. Systematics includes the following:
Identification
It aims at finding the correct name and appropriate position of an organism. The morphological and anatomical characters are examined for proper identification.
Classification
It is almost impossible to study all the living organisms. So, it is necessary to devise some means to make this possible. This can be done by classifying the organisms.
Thus, classification is the process by which organisms are grouped into categories based on some easily observable characters.
Biological classification is the scientific arrangement of organisms in a hierarchy of groups and sub-groups on the basis of similarities and differences in their traits.
Advantages of Classification
(a) It helps to identify an organism easily.
(b) New organisms easily get correct place in their respective groups.
(c) It makes study of fossils easy.
(d) It also helps in building evolutionary pathways.
(e) It becomes easy to know the features of whole group by studying one or two organisms of the group.
Thus, based on these characteristics, all living organisms are classified into different taxa.
Nomenclature

Nomenclature is the system of naming living organism in a way that a particular organism is known by the same name all over the world.
i. Common Names
The common names or vernacular names are the local names given to an organism in a specific language in a particular region. There are different names of a same organism in different regions even with in a country.
Advantages of Common Names
(a) Common names are easy to pronounce and are short, e.g., Cat or billi.
(b) People are familiar to these names since childhood.
(c) They are based on some features of organisms, e.g., Cowa (crow—Caawn-Caawn sound).
Dis-Advantages of Common Names
(a) All the organisms cannot be named by this method as there are organism of different sizes and shapes.
e.g., Microbes.
(b) An organism may have several names in a given language, e.g., 8 Hindi names of prickly poppy and water lily has 15 English names.
(c) A common names may have different meanings in different countries, e.g., Maize, means wheat and other grains in USA and it is called corn in common wealth countries.
(d) Common names may have little relevance, e.g., Lady’s finger (okra), widows tears (Tradescantia-Rhoeo), etc.
(e) Common names may be incorrect, e.g., Jelly fish (a coelenterate), silverfish (an arthropod), starfish (an echinoderm) are not real fishes.
(f) These names are not useful for scientific studies.
ii. Scientific Names
A scientific name is given by biologists. These names represent a particular organism in every part of the world. The system of providing scientific names is called binomial nomenclature.
The scientific names must be
(a) acceptable in every part of the world.
(b) assigned on agreed principles and criteria.
(c) different for each species and not used for other organisms earlier.
Binomial Nomenclature
Binomial nomenclature was developed by Carolus Linnaeus in 1751 (Philosphica Botanica). All scientific names for animals under binomial nomenclature were given by Linnaeus in the tenth edition of his book Systerna Naturae (1758). Linnaeus named plants according to binomial nomenclature in his book Species Plantarum (1753). Binomial nomenclature is the system of providing distinct and appropriate names to organisms, each consisting of two words, first generic name {i.e., name of genus) and second specific epithet (i.e., name of species).
For example, Scientific name of mango is written as Mangifera indica. In this name, Mangifera represents the genus and indica is a particular species or specific epithet.
Rules of Binomial Nomenclature
Rules of binomial nomenclature were initially framed by Linnaeus in his books, Species Plantarum and Systema Naturae.
The rules were revised again by the following nomenclature codes
(i) International Code for Botanical Nomenclature (ICBN).
(ii) International Code of Zoological Nomenclature (ICZN).
(iii) International Code of Bacteriological Nomenclature (ICBN).
(iv) International Code of Viral Nomenclature (ICVN).
(v) International Code of Nomenclature for Cultivated Plants (ICNCP).
The rules framed by Linnaeus and by these codes are as follows
(i) The names are generally in Latin and written in italics. They are Latinised or derived from Latin irrespective of their origin.
(ii) The first word in a biological name represent the genus while, the second component denotes the specific epithet.
(iii) Both the words in a biological name, when handwritten are separately underlined or printed in italics to indicate their Latin origin.
(iv) The first word denoting the genus starts with capital letter while, the specific epithet starts with a small letter, e.g., Mangifera indica.
(v) Generic and common names may be same, e.g., Gorilla gorilla.
(vi) No names are recognised prior to those used by Linnaeus in 1753 for plants in Species Plantarum and in 1758 for animals in the 10th edition of Systema Naturae.
(vii) The name of categories higher than the rank of genus are not printed in italics. Bold letters can, however be used.
(viii) When a species is transferred or revised, the name of the original worker is retained but in parenthesis, e.g., Syzygium cumini (L) Skeels.
Advantages of Binomial Nomenclature
(i) Binomial names are universally acceptable and recognised.
(ii) They remain same in all languages.
(iii) The names are small and comprehensive.
(iv) There is a mechanism to provide a scientific name to every newly discovered organism.
(v) The names indicate relationship of a species with other species present in the same genus.
(vi) A new organism can be easily provided with a new scientific name.
Taxonomy
It is the science of identification, classification and nomenclature. Based on their special / characteristics, all living organisms can be classified into different taxa. This process of classification is called taxonomy. Carolus Linnaeus is known as father of taxonomy.
The basis of modern taxonomy studies are external and internal structure (comparative morphology), along with the structure of cells (cytology), development process (embryology) and ecological information of organisms (ecology). It provide information according to similarities, dissimilarities and evolutionary relationships of various organisms.
The basic processes for taxonomic studies are
(i) Organisms are described on the basis of morphology and other characteristics.
(ii) The description of characteristics helps in the placement of the organism in various taxa.
(iii) A new taxon can be framed if the organism is different from the existing taxa.
(iv) The correct naming of an organism can be done after placing it in various taxon. A new organism can be given a new name after following the standardized rules.
Classical Taxonomy (Old Taxonomy)
The concept of classical or old taxonomy exists since, the time of Aristotle and Theophrastus and continued up to Linnaeus. It states that 4 .
(i) Species is the basic unit of taxonomy, that can be described on the basis of one or few preserved specimens.
(ii) Species are fixed and do not change with time.
(iii) A species is delimited based on morphological features.
(iv) Organisms are classified on the basis of some limited features such as root modification, leaf venation, floral structures, number of cotyledons in case of plants.
Due to the limited number of groups, many organisms could not be classified correctly. This finally led to artificial system of classification.

Modern Taxonomy (New Taxonomy)
The concept of modern taxonomy was given by Julian Huxley (1940). It uses evidences from all the areas of biology like morphology, anatomy, biochemistry, cell biology, physiology, genetics, evolution, etc.
The modem taxonomy is based on the following features
(i) The studies are done on a huge number of organisms based on all the variations.
(ii) Study is also focused on sub-species, varieties, races and populations.
(iii) Species are not isolated. They are related by common descent and vary from them due to accumulation of variations.
(iv) Species is considered as dynamic and ever-changing entity.
(v) Biological delimitation includes various branches of systematics, e.g., Cytotaxonomy, experimental taxonomy, numerical taxonomy, chemotaxonomy, etc. This led to the development of phylogenetic system or cladistics of classification.
Taxonomic Categories
Classification is not a single step process. It involves hierarchy of steps in which each step represents a rank or category. Since, the category is a part of overall taxonomic arrangement, it is called the taxonomic category and all categories together constitute the taxonomic hierarchy.
Taxon
Each category, referred to as a unit of classification, in fact, represents a rank and is commonly termed as taxon (Pi. taxa). The term Taxon was first introduced by ICBN during 1956.
According to Mayr (1964) taxon is a group of any rank that is sufficiently distinct to be worthy of being assigned a definite category. In simple words, taxon refers to a group of similar, genetically related individuals having certain characters distinct from those of other groups.
A taxon that includes a common ancestral species and all the species descended from it is called a clade or a monophyletic taxon.
Taxonomic Hierarchy
The taxonomic hierarchy is the system of arranging taxonomic categories in a descending order. It was first introduced by Linnaeus (1751) and hence, it is also known as Linnaen hierarchy.
Groups represent category and category further denotes rank. Each rank or taxon represents a unit of classification.
These taxonomic groups/categories are distinct biological entities and not merely morphological aggregates.
Obligate/Common Categories
The taxonomic categories, which are always used in hierarchical classification of organisms are called obligate or common categories.
They are seven in number. In descending order, these are kingdom, phylum or division, class, order, family, genus and species.
All the members of taxonomic categories possess some similar characters, which are different from those of others. The maximum similarity occurs in species, which is also the lowest category in the hierarchy of categories. Similarity of characters decreases with the rise in hierarchy.
i. Species
Taxonomic studies consider a group of individual organisms with fundamental similarities as a species (John Ray).
Species is considered as the lowest or basic taxonomic category, which consists of one or more individuals of a populations that resemble one another more closely than individuals of other species. The members of species interbreed freely and are reproductively isolated from others. For example, Mangifera indica (mango), Solarium tuberosum (potato) and Panthera leo (lion).
All the three names indica, tuberosum and leo represent the specific epithets while, the first words Mangifera, Solanum and Panthera are genera and represents another higher level of taxon or category.
Each genus may have one or more than one specific epithets representing different organisms, but having morphological similarities. For example, Panthera has another specific epithet called tigris and Solanum includes species like nigrum and melongena.
ii. Genus
Genus (John Ray) comprises a group of related species, which has more characters common in comparison to species of other genera. In other words, genera are aggregates of closely related species.
iii. Family
Family (John Ray) is a group of related genera with less number of similarities as compared to genus and species. All the genera of a family have some common or correlated features. They are separable from genera of a related family by important differences in both vegetative and reproductive features.
A plant family ends in a suffix -aeae and sub-family -oideae. While, an animal family has a suffix -idae and sub-family -inae.

iv. Order
An order (Linnaeus) is a group of one or more related families that possess some similar correlated characters, which are lesser in number as compared to a family or genera.
Plants and Animal Orders with their Respective Families
Order Animals and Families
Carnivora Canidae (dog, wolf and fox), Felidae (cat, leopard, tiger and lion), Ursidae (bear) and Hyaenidae (hyaena)
Polemoniales Solanaceae (potato and tomato), Convolvucaceae (sweet potato and morning glory), Polemoniaceae (herbs, shrubs and small trees) and Hydrophyllaceae (water leaf).
Primates Lemuridae (lemurs), Cebidae (new world monkeys), Pongidae (apes) and Hominidae (humans).
v. Class
Class (Linnaeus) is a major category, which includes related orders. For example, order-Primata comprises monkey, gorilla & gibbon and is placed in class—Mammalia along with order—Carnivora that includes animals like tiger, cat and dog.
Class-Mammalia has other orders also.
vi. Phylum or Division
Phylum or Division (Cuvier, Eichler) is a taxonomic category higher than class and lower” in rank to kingdom. The term Phylum is used for animals, while division is commonly employed for plants.
It consists of more than one class having some similar corelated characters.
For example, Phylum— Chordata of animals contain following classes, e.g., Pisces, amphibians, reptiles, aves and mammals.
vii. Kingdom
It is known to be the highest category in taxonomy. This includes all the organisms, which share a set of distinguished characters. For example, all the animals belonging to various phyla are assigned the highest category called kingdom.
For example, Animalia in the classification system of animals. Similarly, all the plants are kept in kingdom—Plantae.
RH Whittaker. (1969) assigned five kingdom classification of organisms.
These are Monera, Protista, Fungi, Plantae and Animalia.
Intermediate Categories
The taxonomic categories from species to kingdom are broad categories or obligate categories. However, taxonomists have also developed sub-categories in this hierarchy to facilitate more sound and scientific placement of various taxa. These sub-categories are sub-species (or varieties), sub-genera, sub-families, sub-orders, sub-classes and sub-phyla.
These sub-categories are referred to as intermediate categories.
Taxonomical Aids
Taxonomical aids are techniques and procedures to store information as well as specimens or identification and classification of organisms.
The taxonomic studies of various plants, animals and other organisms are useful in areas like agriculture, forestry, industry and knowing our bioresources. All these studies need correct identification and classification of organisms. Identification of organisms requires intensive laboratory and field studies. The collection of actual specimens of plants and animal species, knowing their habitats and other traits are essential and are the prime source of taxonomic studies. All this information is used in classification of an organism and is also stored along with the specimens. Sometimes, specimens are also preserved for future studies.
Some of the taxonomical aids developed by Biologists include Herbarium, Botanical gardens, Museum, Zoological parks, Key, etc.
1. Herbarium
Herbarium (Pi. Herbaria) is a store house of collected plant specimens that are dried, pressed and preserved on sheets. These sheets are arranged further according to a universally accepted system of classification. The institutes and universities maintain their own herbarium by collecting specimens from local and far away places.
Uses of Herbaria
The uses of herbaria are listed below
(a) These are used for identification of plants.
(b) Compilation of floras, monographs and manuals are mainly based on the specimens in herbaria.
(c) Herbaria are useful in locating wild varieties and relatives of economically important plants.
(d) They help in knowing the morphological variations found in species.
(e) Herbaria are useful for research in plant taxonomy, morphology, ecological distribution, etc.

2. Botanical Gardens
Botanical gardens are specialised gardens that have collections of living plants for reference. These gardens generally have facilities like library, laboratory, herbarium and museum. The botanical gardens are maintained by government, semi-government and other private organisations. Botanists and gardeners look after plants in botanical gardens.
Role of Botanical Gardens
A botanical garden has following important roles
(a) Botanical gardens have aesthetic appeal and provide recreation facility to people.
(b) A wide variety of plant species grow there, so they provide ready material for research.
(c) These gardens also play an important role in conservation of endangered plant species and genetic diversity.
(d) There are more than 500 botanical gardens all over the world. These provide free exchange of seeds.
(e) These improve the environment, provide greenery, help in creating pollution free environment and some serves as habitat for animals.
Knowledge Plus
Indian Botanical Garden-Largest Botanical Garden of Asia.
First Botanical Garden-Pisa Botanical Garden, Italy established by Luca Glini (1490-1556).
3. Museums
Museum is a place for collections of preserved plants and animal specimens for study and reference. The universities and educational institutes maintain their own museums in their botany and zoology departments. Plants, which cannot be kept in herbaria are preserved in museums.

For example, algae, fungi, mosses, ferns, fruits, etc. Specimens are preserves in containers or jars in preservative solutions. Plant and animal specimens may also be preserved as dry specimens. Insects are preserved in insect boxes after collecting killing and pinning. While, the larger animals are stuffed and preserved in skeletal forms.
4. Zoological Parks
Zoological parks or zoo are the places where wild animals are kept in protected environments under human care and which enable us to learn about their food habits and behaviour. Zoological parks provide natural habitat to the animals.
In India there are about 200 zoological parks. These zoos are managed by the Central Zoo Authority of India. The World Zoo Conservation Strategy (WZCS) refer to all these zoological institutions as zoos.
Role of Zoological Parks
(a) The zoological parks increase understanding of wildlife.
(b) These are the centres for recreation and education.
(c) Zoos are the centres for conservation of threatened and rare animal species.
(d) These provide sites for ex situ breeding of endangered animals. conservation through captive breeding of endangered animals.
5. Key
Key is also a taxonomical aid used for identification of plants and animals based on the similarities and dissimilarities.
It helps in the identification of plants and animals by selecting and eliminating the characters according to their presence or absence in the organism under study.
The keys generally use two contrasting characters called couplet. This results in acceptance of one present in organism and rejection of the other. Each statement in the key is called a lead.
These taxonomic keys are of two types
Indented Key
The indented key or yolked key provides a sequence of choices between two or more characteristics. By careful selection of characters at each sub-division, the exact name of the organism can be arrived at.
Bracketed Key
The bracketed key also uses contrasting characters like the indented key. But in, these characters are not separated by intervening sub-dividing characters. Each character in this case is given a number in brackets.
Other Means of Recording Descriptions
Apart from the all mentioned means of keeping records of description. Some other means are also present.
These are of following types
Flora
Floras are the important resource that provide information on the taxonomy, nomenclature and descriptive data for the taxa covered.
The floras also include information on the biology, distribution and habitat preferences of the taxa, as well as illustrations, identification keys and other notes. These provide index to the plant species found in a particular area.
Manuals and Catalogues
These are other means of recording descriptions. They also help in correct identification. Manuals are useful in providing information for identification of names of species found in an area.
Monograph
A monograph is a comprehensive treatment of a taxon in biological taxonomic studies. These contain information on any one taxon. Monographs revise all known species within a group, add any newly discovered species, collect and organise available information on the ecological associations, geographic distributions and morphological variations within the group.
The first ever monograph of a plant taxon was given in Robert Morison (1672) Plantarum Umbelliferarum Distributio Nova.