Redox Reactions Class 11 Notes Chemistry
Introduction
Redox reaction is related to gain or loss of electrons. Reaction in which oxidation and reduction takes place simultaneously is called redox reaction. This chapter deals with problems based on redox reactions, oxidation number and balancing of redox reactions by ion, electron method and oxidation number method.
Oxidation Reactions
Oxidation is defined as the addition of oxygen/electronegative element to a substance or rememoval of hydrogen/ electropositive element from a susbtance.
2Mg(s) + O2(g) ⟶ 2MgO(s)
Mg(s) + Cl2(g) ⟶ MgCl2(s)
Reduction Reactions
Reduction is defined as the memoval of oxygen/electronegative element from a substance or addition of hydrogen or electropositive element to a substance.
2FeCl3(aq) + H2(g) ⟶ 2FeCl2(aq) + 2HCl(aq)
2HgO(s) ⟶ 2Hg(l) + O2(g)
Redox Reactions
Reaction in which oxidation and reduction takes place simultaneously is called redox reaction. Oxidation and reduction are complementary to each other, one cannot take place alone. So both oxidation and reduction will occur simultaneously. It is obvious that if a substance takes electrons there must be another substance to give up these electrons.
2FeCl3 + SnCl2 ⟶ 2Fecl2 + SnCl4
Oxidation Number or Oxidation State
Oxidation number for an element is the arbitrary charge present on one atom when all other atoms bonded to it are removed. For example, if we consider a molecule of HCl, the Cl atom is more electronegative than H-atom, therefore, the bonded electrons will go with more electronegative chlorine atom resulting in formation of H+ and Cl– ions. So oxidation number of H and Cl in HCl are +1 and –1 respectively.
The following points are important to determine the oxidation number of an element.
- The oxidation number of an atom in pure elemental form is considered to be zero. e.g., H2, O2, Na, Mg
- Oxidation number of any element in simple monoatomic ion will be equal to the charge on that ion, for example, oxidation number of Na in Na+ is +1.
- Oxidation number of fluorine in its compound with other elements is always –1.
- Oxidation number of oxygen is generally –2 but in case of peroxide (H2O2) oxygen has oxidation number –1. In a compound OF2 the oxidation number of oxygen is +2.
- The oxidation number of alkali metals (Na, K) and alkaline earth metals (Ca, Mg) are +1 and +2 respectively.
- The oxidation number of halogens is generally –1 when they are bonded to less electronegative elements.
- Oxidation number of hydrogen is generally +1 in most of its compounds but in case of metal hydride (NaH, CaH2) the oxidation number is hydrogen is –1.
- The algebraic sum of the oxidation numbers of all the atoms in a neutral compound is zero. In an ion, the algebraic sum of oxidation number is equal to the charge on that ion.
Oxidising and Reducing Agent
A substance which undergoes oxidation acts as a reducing agent while a substance which undergoes reduction acts as an oxidising agent. For example, we take a redox reaction,
Zn + Cu2+ ⟶ Zn2+ + Cu
In this reaction, Zn is oxidised to Zn2+ so Zn is reducing agent and Cu2+ is reduced to Cu so Cu2+ is an oxidising agent.
Types of Redox Reactions
1. Combination reactions
A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is:
A + B ⟶ AB
Na(s) + Cl2(g) ⟶ 2NaCl(s)
2. Decomposition reactions
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a decomposition reaction is:
AB ⟶ A + B
2HgO(s) ⟶ 2Hg(l) + O2(g)
3. Displacement reactions
Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound.
CuSO4(aq) + Zn(s) ⟶ ZnSO4(aq) + Cu
4. Disproportionation reactions
The reactions in which a single reactant is oxidized and reduced is known as Disproportionation reactions. The disproportionation reaction is given below
2H2O2 ⟶ 2H2O + O2
Balancing of Redox Reactions
(a) Oxidation Number Method
In this method number of electrons lost in oxidation must be equal to number of electrons gained in reduction. Following rules are followed for balancing of reactions:
- Write the skeletal equation of all the reactants and products of the reaction.
- Indicate the oxidation number of each element and identify the elements undergoing change in oxidation number.
- Equalize the increase or decrease in oxidation number by multiplying both reactants and products undergoing change in oxidation number by a suitable integer.
- Balance all atoms other than H and O, then balance O atom by adding water molecules to the side short of O-atoms.
- In case of ionic reactions(a) For acidic medium : First balance O atoms by adding H2O molecules to the side deficient in O atoms and then balance H-atoms by adding H+ ions to the side deficient in H atoms.(b) For basic medium : First balance O atoms by adding H2O molecules to whatever side deficient in O atoms. The H atoms are then balanced by adding H2O molecules equal in number to the deficiency of H atoms and an equal number of OH– ions are added to the opposite side of the equations.

(b) Ion-Electron Method
- Write the skeleton equation and indicate the oxidation number of all the elements which appear in the skeletal equation above their respective symbols.
- Find out the species which are oxidised and which are reduced.
- Split the skeleton equation into two half reactions, i.e., oxidation half reaction and reduction half reaction.
- Balance the two half reaction equations separately by the rules described below(i) In each half reaction, 1st balance the atoms of the elements which have undergone a change in oxidation number.(ii) Add electrons to whatever side is necessary to make up the difference in oxidation number in each half reaction.(iii) Balance oxygen atoms by adding required number of H2O molecules to the side deficient in O atoms.(iv) In the acidic medium, H atoms are balanced by adding H+ ions to the side deficient in H-atoms. However, in the basic medium, H atoms are balanced by adding H2O molecules equal in number to the deficiency of H atoms and an equal number OH– ions are included in the opposite side of the equation.
- The two half reactions are then multiplied by suitable integers so that the total number of electrons gained in one half of the reaction is equal to the number of electrons lost in the other half reaction. The two half reactions are then added up.
- To verify whether the equation thus obtained is balanced or not, the total charge on either side of the equation must be equal.
Galvanic Cell and Electrode Potential
A galvanic cell or voltaic cell is simple electrochemical cell in which a redox reaction is used to convert chemical energy into electrical energy. It means electricity can be generated with the help of redox reaction in which oxidation and reduction takes place in two separate compartments. Each compartment consists of a metallic conductor and dipped in suitable electrolytic solution of same metal. Metallic rod acts as electrode.
The compartment having electrode dipped in solution of electrolyte is known as half cell and a half cell has a redox couple. A redox couple means a solution having reduced and oxidised form of a substance together, taking part in oxidation or reduction half reaction. It is depicted as M+n / M i.e., oxidised form / reduced form. To prepare a galvanic cell two half cells are externally connected through a conducting wire and internally through salt bridge.
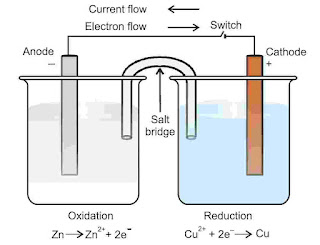
Anodic oxidation : Zn2 ⟶ Zn+2(aq) + 2e(s)
Cathodic reduction : Cu+2(aq) + 2e ⟶ Cu(s)
Net reaction : Zn(s) + Cu+2(aq) ⟶ Zn+2(aq) + Cu(s)
This cell can be briefly presented in one line, known as cell notation i.e.,
Zn | Zn+2 || Cu+2 | Cu
Discover more from EduGrown School
Subscribe to get the latest posts sent to your email.
