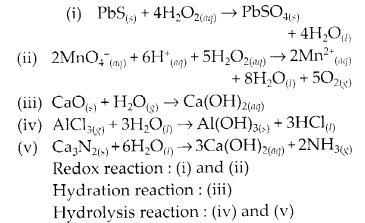NCERT Exercises
Question 1.
A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The
volume of the container is suddenly increased.
(a) What is the initial effect of the change on vapour pressure?
(b) How do rates of evaporation and condensation change initially?
(c) What happens when equilibrium is restored finally and what will be the final vapour pressure?
Solution.
(a) Vapour pressure decreases since equilibrium is disturbed and the rate of condensation falls considerably.
(b) Rate of evaporation increases while that of condensation falls initially.
(c) On restoration of the equilibrium again, the vapour pressure becomes the same.
Question 2.
What is Kc for the following equilibrium when the equilibrium concentration of each substance is : [SO2] = 0.60 M, [O2] = 0.82 M and [SO2] = 1.90 M
2SO2(g) + O2(g) ⇌ 2SO3(g)
Solution.
2SO2(g) + O2(g) ⇌ 2SO3(g)
Applying law of chemical equilibrium,
![{ K }_{ c }=\frac { { \left[ { SO }_{ 3 } \right] }^{ 2 } }{ { \left[ { SO }_{ 2 } \right] }^{ 2 }\left[ { O }_{ 2 } \right] } =\frac { { (1.9) }^{ 2 } }{ { (0.6) }^{ 2 }{ (0.82) } } =12.229L\quad { mol }^{ -1 } { K }_{ c }=\frac { { \left[ { SO }_{ 3 } \right] }^{ 2 } }{ { \left[ { SO }_{ 2 } \right] }^{ 2 }\left[ { O }_{ 2 } \right] } =\frac { { (1.9) }^{ 2 } }{ { (0.6) }^{ 2 }{ (0.82) } } =12.229L\quad { mol }^{ -1 }](https://s0.wp.com/latex.php?latex=%7B+K+%7D_%7B+c+%7D%3D%5Cfrac+%7B+%7B+%5Cleft%5B+%7B+SO+%7D_%7B+3+%7D+%5Cright%5D+%7D%5E%7B+2+%7D+%7D%7B+%7B+%5Cleft%5B+%7B+SO+%7D_%7B+2+%7D+%5Cright%5D+%7D%5E%7B+2+%7D%5Cleft%5B+%7B+O+%7D_%7B+2+%7D+%5Cright%5D+%7D+%3D%5Cfrac+%7B+%7B+%281.9%29+%7D%5E%7B+2+%7D+%7D%7B+%7B+%280.6%29+%7D%5E%7B+2+%7D%7B+%280.82%29+%7D+%7D+%3D12.229L%5Cquad+%7B+mol+%7D%5E%7B+-1+%7D+&bg=ffffff&fg=000&s=0)
Question 3.
At a certain temperature and total pressure of 105 Pa, iodine vapour contains 40% by volume of I atoms. I2(g) ⇌ 2l(g)Calculate Kp for the equilibrium.
Solution.


Question 4.
Write the expression for the equilibrium constant, Kc for each of the following reactions:

Solution.


Question 5.
Find out the value of Kcof each of the following equilibria from the value of Kp:

Solution.

Question 6.
For the following equilibrium, Kc= 6.3 x 1014 at 1000 K. NO(g) + O3(g) ⇌ NO2(g) + O2(g) the forward and reverse reactions in the equilibrium are elementary bimolecular reactions. What is Kc, for the reverse reaction?
Solution.
NO(g) + O3(g) ⇌ NO2(g) + O2(g)
![{ K }_{ c }=\frac { \left[ { NO }_{ 2 } \right] \left[ { O }_{ 2 } \right] }{ \left[ NO \right] \left[ { O }_{ 3 } \right] } =6.3\times { 10 }^{ 14 } { K }_{ c }=\frac { \left[ { NO }_{ 2 } \right] \left[ { O }_{ 2 } \right] }{ \left[ NO \right] \left[ { O }_{ 3 } \right] } =6.3\times { 10 }^{ 14 }](https://s0.wp.com/latex.php?latex=%7B+K+%7D_%7B+c+%7D%3D%5Cfrac+%7B+%5Cleft%5B+%7B+NO+%7D_%7B+2+%7D+%5Cright%5D+%5Cleft%5B+%7B+O+%7D_%7B+2+%7D+%5Cright%5D+%7D%7B+%5Cleft%5B+NO+%5Cright%5D+%5Cleft%5B+%7B+O+%7D_%7B+3+%7D+%5Cright%5D+%7D+%3D6.3%5Ctimes+%7B+10+%7D%5E%7B+14+%7D&bg=ffffff&fg=000&s=0)
For the reverse reaction,
![{ K }_{ c }^{ ` }=\frac { \left[ NO \right] \left[ { O }_{ 3 } \right] }{ \left[ { NO }_{ 2 } \right] \left[ { O }_{ 2 } \right] } =\frac { 1 }{ { K }_{ c } } =\frac { 1 }{ 6.3\times { 10 }^{ 14 } } =1.587\times { 10 }^{ -15 } { K }_{ c }^{ ` }=\frac { \left[ NO \right] \left[ { O }_{ 3 } \right] }{ \left[ { NO }_{ 2 } \right] \left[ { O }_{ 2 } \right] } =\frac { 1 }{ { K }_{ c } } =\frac { 1 }{ 6.3\times { 10 }^{ 14 } } =1.587\times { 10 }^{ -15 }](https://s0.wp.com/latex.php?latex=%7B+K+%7D_%7B+c+%7D%5E%7B+%60+%7D%3D%5Cfrac+%7B+%5Cleft%5B+NO+%5Cright%5D+%5Cleft%5B+%7B+O+%7D_%7B+3+%7D+%5Cright%5D+%7D%7B+%5Cleft%5B+%7B+NO+%7D_%7B+2+%7D+%5Cright%5D+%5Cleft%5B+%7B+O+%7D_%7B+2+%7D+%5Cright%5D+%7D+%3D%5Cfrac+%7B+1+%7D%7B+%7B+K+%7D_%7B+c+%7D+%7D+%3D%5Cfrac+%7B+1+%7D%7B+6.3%5Ctimes+%7B+10+%7D%5E%7B+14+%7D+%7D+%3D1.587%5Ctimes+%7B+10+%7D%5E%7B+-15+%7D&bg=ffffff&fg=000&s=0)
Question 7.
Explain why pure liquids and solids can be ignored while writing the equilibrium constant expression.
Solution.
For the concentration of pure solid or pure liquid,


Since density of pure solid or liquid is constant at constant temperature and molar mass is also constant therefore, their molar concentrations are constant and are included in the equilibrium constant.
Question 8.
Reaction between N2 and O2 takes place as follows:
2N2(g) + O2(g) ⇌ 2N2O(g)
If a mixture of 0.482 mol of N2 and 0.933 mol of O2 is placed in a 10 L reaction vessel and allowed to form N2O at a temperature for which Kc = 2.0 x 10-37, determine the composition of equilibrium mixture.
Solution.

Question 9.
Nitric oxide reacts with Br2 and gives nitrosyl bromide as reaction given below:
2NO(g) + Br2(g) ⇌ 2NOBr(g) When 0.087 mol of NO and 0.0437 mol of Br2 are mixed in a closed container at constant temperature, 0.0518 mol of NOBr is obtained at equilibrium. Calculate equilibrium amount of NO and Br2.
Solution.

Question 10.
At 450 K, Kp = 2.0 x 1010.bar for the given reaction at equilibrium.
2SO2(g)+ O2(g) ⇌ 2SO3(g)
What is Kc at this temperature?
Solution.

Question 11.
A sample of HI(g) is placed in a flask at a pressure of 0.2 atm. At equilibrium partial pressure of HI(g) is 0.04 atm. What is Kp for the given equilibrium ?
2HI(g)  H2(g) + I2(g)
H2(g) + I2(g)
Solution.

Question 12..
AA mixture of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3 is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant Kc for the reaction given as follows :
N2(g) + 3H2(g)  2NH3(g) is 1.7 × 10-2.
2NH3(g) is 1.7 × 10-2.
Is this reaction at equilibrium ? If not, what is the direction of net reaction ?
Solution.

Question 13.
The equilibrium constant expression for a gas reaction is,

Write the balanced chemical equation corresponding to this expression.
Solution.
Balanced chemical equation for the reaction is
4 NO(g) + 6 H2O(g)  4NH3(g) + 5 O2(g)
4NH3(g) + 5 O2(g)
Question 14.
If 1 mole of H2O and 1 mole of CO are taken in a 10 litre vessel and heated to 725 K, at equilibrium point 40 percent of water (by mass) reacts with carbon monoxide according to equation,
H2O(g) + CO(g)  H2(g) + CO2(g)
H2(g) + CO2(g)
Calculate the equilibrium constant for the reaction.
Solution.

Question 15.
At 700 K, the equilibrium constant for the reaction H2(g) + I2(g)  2HI(g) is 54.8. If 0.5 mol L-1 of HI(g) is present at equilibrium at 700 K, what are the concentration of H2(g) and I2(g) ? Assume that we initially started with HI(g) and allowed it to reach equilibrium at 700 K.
2HI(g) is 54.8. If 0.5 mol L-1 of HI(g) is present at equilibrium at 700 K, what are the concentration of H2(g) and I2(g) ? Assume that we initially started with HI(g) and allowed it to reach equilibrium at 700 K.
Solution.

Question 16.
What is the equilibrium concentration of each of the substances in the equilibrium when the initial concentration of ICl was 0.78 M ?
2ICl(g)  I2(g) + Cl2(g) ; Kc = 0.14
I2(g) + Cl2(g) ; Kc = 0.14
Solution.


Question 17.
Kp = 0.04 atm at 898 K for the equilibrium shown below. What is the equilibrium concentration of C2H6 when it is placed in a flask at 4 atm pressure and allowed to come to equilibrium.
C2H6(g)  C2H4(g) + H2(g)
C2H4(g) + H2(g)
Solution.

Question 18.
The ester, ethyl acetate is formed by the reaction of ethanol and acetic acid and the equilibrium is represented as :
CH3COOHl + C2H5OHl  CH3COOC2H5l + H2Ol
CH3COOC2H5l + H2Ol
(i) Write the concentration ratio (concentration quotient) Q for this reaction. Note that water is not in excess and is not a solvent in this reaction.
(ii) At 293 K, if one starts with 1.000 mol of acetic acid and 0.180 mol of ethanol, there is 0.171 mol of ethyl acetate in the final equilibrium mixture. Calculate the equilibrium constant.
(iii) Starting with 0.50 mol of ethanol and 1.000 mol of acetic acid and maintaining it at 293 K, 0.214 mol of ethyl acetate
is found after some time. Has equilibrium been reached ?
Solution.



Question 19.
A sample of pure PCl5 was introduced into an evacuated vessel at 473 K. After equilibrium was reached, the concentration of PCl5 was found to be 0.5 x 10-1 mol L-1. If Kc is 8.3 × 10-3, what are the concentrations of PCl3 and Cl2 at the equilibrium ?

Solution.

Question 20.
One of the reactions that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and CO2
FeO(s) + CO(g)  Fe(s) + CO2(g); Kp = 0.265 atm at 1050 K
Fe(s) + CO2(g); Kp = 0.265 atm at 1050 K
What are the equilibrium partial pressures of CO and CO2 at 1050 K if the initial pressures are :Pco = 1.4 atm and Pco2 = 0.80 atm ?
Solution.

Question 21.
Equilibrium constant Kc for the reaction, N2(g) + 3H2(g)  2NH3(g) at 500 K is 0.061. At particular time, the analysis shows that the composition of the reaction mixture is : 3.0 mol L-1 of N2 , 2.0 mol L-1 of H2,0.50 mol L-1 of NH3. Is the reaction at equilibrium ? If not, in which direction does the reaction tend to proceed to reach the equilibrium ?
2NH3(g) at 500 K is 0.061. At particular time, the analysis shows that the composition of the reaction mixture is : 3.0 mol L-1 of N2 , 2.0 mol L-1 of H2,0.50 mol L-1 of NH3. Is the reaction at equilibrium ? If not, in which direction does the reaction tend to proceed to reach the equilibrium ?
Solution.

Question 22.
Bromine monochloride (BrCl) decomposes into bromine and chlorine and reaches the equilibrium :
2BrCl(g)  Br2(g) + Cl2(g)
Br2(g) + Cl2(g)
For which Kc is 32 at 500 K. If initially pure BrCl is present at a concentration of 3.3× 10-3 mol L-1, what is its molar concentration in the mixture at equilibrium ?
Solution.


Question 23.
At 1127 K and 1 atmosphere pressure, a gaseous mixture of CO and CO2 in equilibrium with solid carbon has 90.55% by mass.
C(s) + CO2(g)  2CO(g)
2CO(g)
Calculate Kc for the reaction at the above temperature.
Solution.

Question 24.

Solution.

Question 25.
Does the number of moles of reaction products increase, decrease or remain same when each of the following equilibria is subjected to a decrease in pressure by increasing the volume ?
(a) PCl5(g)  PCl3(g) + Cl2
PCl3(g) + Cl2
(b) CaO(s) + CO2(g)  CaCO3(s)
CaCO3(s)
(c) 3Fe(s) + 4H2O(g)  Fe3O4(s) + 4H2(g)
Fe3O4(s) + 4H2(g)
Solution.
Applying Le Chatelier’s principle, on decreasing the pressure, equilibrium shifts to the direction in which pressure increases, i.e., number of moles of gaseous substances is more. Thus, moles of reaction products will increase in reaction (a), decrease in reaction (b) and remain same (np, = nr gaseous) in reaction (c).
Question 26.
Which of the following reactions will get affected by increase in pressure ? Also mention whether the change will cause the reaction to go in the forward or backward direction.

Solution.

Question 27.
The equilibrium constant for the following reaction is 1.6 x 105 at 1024 K.
H2(g) + Br2(g)  2HBr(g)
2HBr(g)
Find the equilibrium pressure of all gases if 10.0 bar of HBr is introduced into a sealed container at 1024K.
Solution.

Question 28.
Dihydrogen gas is obtained from the natural gas by partial oxidation with steam as per following endothermic reaction :
CH4(g) + H2O(g)  CO(g) + 3H2(g)
CO(g) + 3H2(g)
(a) Write the expression for Kp for the above reaction
(b) How will the value of Kp and composition of equilibrium mixture be affected by :
(i) increasing the pressure
(ii) increasing the temperature
(iii) using a catalyst ?
Solution.

(b)
- By Le Chatelier’s principle, on increasing pressure, equilibrium will shift in the backward direction where number of moles decreases.
- As the given reaction is endothermic, by Le Chatelier ‘sprinciple, equilibrium will shift in the forward direction with increasing temperature.
- Equilibrium composition will not be disturbed by the presence of catalyst but equilibrium will be attained quickly.
Question 29.
Describe the effect of :
(a) addition of H2
(b) addition of CH3OH
(c) removal of CO
(d) removal of CH3OH on the equilibrium of the reaction:
2H2(g) + CO(g)  CH3OH ?
CH3OH ?
Solution.
2H2(g) + CO(g)  CH3OH Effect of
CH3OH Effect of
(a) addition of H2 : The equilibrium will shift in the forward direction.
(b) addition of CH3OH : The equilibrium will shift in the backward direction.
(c) removal of CO : The equilibrium will shift in the backward direction.
(d) removal of CH3OH : The equilibrium will shift in the forward direction.
Question 30.
At 473 K, the equilibrium constant Kc for the decomposition of phosphorus pentachloride (PCl5) is 8.3 × 10-3. If decomposition proceeds as :
PCl5(g)  PCl3(g) + Cl2(g) ; ∆H = + 124.0 kJ mol-1
PCl3(g) + Cl2(g) ; ∆H = + 124.0 kJ mol-1
(a) Write an expression for Kc for the reaction.
(b) What is the value of Kc for the reverse reaction at the same temperature ?
What would be the effect on Kc if
(i) more PCl5 is added
(ii) pressure is increased
(iii) the temperature is increased?
Solution.

Question 31.
Dihydrogen gas used in Haber’s process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H2. In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction.
CO(g) + H2O(g)  CO2(g) + H2(g)
CO2(g) + H2(g)
If a reaction vessel at 400°C is charged with an equimolar mixture of CO and steam so that pco = PH2O = 4.0 bar, what will be the partial pressure of H2 at equilibrium ? Kp = 0.1 at 400°C.
Solution.

Question 32.
Predict which of the following will have appreciable concentration of reactants and products :
(a) Cl2(g)  2Cl(g) ; Kc = 5 ×10-39
2Cl(g) ; Kc = 5 ×10-39
(b) Cl2(g) + 2NO(g)  2NOCl(g) ; Kc = 3.7 × 108
2NOCl(g) ; Kc = 3.7 × 108
(c) Cl2(g) + 2NO2 (g)  2NO2Cl(g) ; Kc = 1.8.
2NO2Cl(g) ; Kc = 1.8.
Solution.
(a) Since the value of Kc is very small
∴ There will be appreciable concentration of the reactants.
(b) Since Kc is very large.
∴ There will be appreciable concentration of the products.
(c) Since Kc = 1.8, the concentration of reactants and products will be in comparable amounts.
Question 33.
The value of Kc for the reaction 3O2(g)  2O3(g) is 2.0 × 1 x 10-50 at 25°C. If equilibrium concentration of O2 in air at 25°C is 1.6 × 10-2, what is the concentration of O2 ?
2O3(g) is 2.0 × 1 x 10-50 at 25°C. If equilibrium concentration of O2 in air at 25°C is 1.6 × 10-2, what is the concentration of O2 ?
Solution.

Question 34.
The reaction CO(g) + 3H2(g)  CH4(g) + H2O(g) is at equilibrium at 1300 K in a 1L flask. It also contain 0.30 mol of CO, 0.10 mol of H2 and 0.02 mol of H2O and an unknown amount of CH4 in the flask. Determine the concentration of CH4 in the mixture. The equilibrium constant, Kc for the reaction at the given temperature is 3.90.
CH4(g) + H2O(g) is at equilibrium at 1300 K in a 1L flask. It also contain 0.30 mol of CO, 0.10 mol of H2 and 0.02 mol of H2O and an unknown amount of CH4 in the flask. Determine the concentration of CH4 in the mixture. The equilibrium constant, Kc for the reaction at the given temperature is 3.90.
Solution.

Question 35.
What is meant by conjugate acid-base pair ? Find the conjugate acid/base for the following species :
HNO2,CN–,HClO4,OH–,CO32-,S2-.
Solution.

Question 36.
Which of the following are Lewis Acids ?
H2O, BF3, H+ and NH4+
Solution.
BF3, H+ and NH4+ are Lewis acids because they can accept a lone pair of electrons.
Question 37.
What will be the conjugate bases for the Bronsted acids ? HF, H2SO4 and  ?
?
Solution.

Question 38.
Write the conjugate acids for the following Bronsted acids.
 , NH3 and HCOO–
, NH3 and HCOO–
Solution.

Question 39.
The species H2O, HCO3–, HSO4– and NH3 can act both as Bronsted acid and base. For each case, give the corresponding conjugate acid and base.
Solution.

Question 40.
Classify the following species into Lewis acids and Lewis bases and show how these can act as Lewis acid/Lewis base ?
(a) OH–
(b) F–
(c) H+
(d) BCl3
Solution.
(a) OH– : OH– is a Lewis base because it can donate lone pair of electrons.
(b) F– : F– is a Lewis base because it can donate lone pair of electrons.
(c) H+ : H+is a Lewis acid because it can accept lone pair of electrons.
(d) BCl3 : is a Lewis acid because it is electron deficient and can accept a lone pair of electrons.
Question 41.
The concentration of hydrogen ions in a sample of soft drink is 3.8 × 10-3 M. What is its pH value ?
Solution.

Question 42.
The pH of soft drink is 3.76. Calculate the concentration of hydrogen ions in it.
Solution.

Question 43.
The ionisation constants of HF, HCOOH and HCN at 298 K are 6.8 x 10-4, 1-8 × 10-4 and 4.8 × 10-9 respectively. Calculate the ionisation constant of the corresponding congugate bases.
Solution.

Question 44.
The ionisation constant of phenol is 1.0 x 10-10. What is the concentration of phenate ion in 0.05 M solution of phenol and pH of solution ? What will be the degree of ionisation if the solution is also 0.01 M in sodium phenate ?
Solution.

Question 45.
The first dissociation constant H2S is 9.1 × 10-8. Calculate the concentration of HS– ions in its 0.1 M solution and how much will this concentration be affected if the solution is 0.1 M in HCl also. If the second dissociation constant of H2S is 1.2 × 10-13, calculate the concentration of S2- ions under both the conditions.
Solution.


Question 46.
The ionization constant of acetic acid is 1.74 × 10-5. Calculate the degree of dissociation of acetic acid in 0.05 M solution. Calculate the concentration of acetate ions in solution and the pH of the solution.
Solution.

Question 47.
It has been found that the pH of 0.01 M solution of organic acid is 4.15. Calculate the concentration of the anion, ionization constant of the acid and the pKa.
Solution.

Question 48.
Assuming complete dissociation, calculate the pH of the following solutions :
(a) 0.003 M HCl
(b) 0.005 M NaOH
(c) 0.002 M HBr
(d) 0.002 M KOH.
Solution.

Question 49.
Calculate the pH of the following solutions :
(a) 2 g of TIOH dissolved in water to give 2 litre of solution.
(b) 0.3 g of Ca(OH)2 dissolved in water to give 500 mL of solution.
(c) 0.3 g of NaOH dissolved in water to give 200 mL of solution.
(d) 1 mL of 13.6 M HCl diluted with water to give 1 litre of solution.
Solution.

Question 50.
The degree of ionization of 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and pKa of bromoacetic acid.
Solution.

Question 51.
The pH of 0-005 M codeine (C18H21NO3) solution is 9.95. Calculate its ionizationconstant and pKb.
Solution.

Question 52.
What is the pH of 0.001 M aniline solution ? The ionization constant of aniline is 4.27 × 10-10. Calculate the degree of ionization of aniline in the solution. Also calculate the ionization constant of the conjugate acid of aniline.
Solution.

Question 53.
Calculate the degree of ionization of 0.05 M acetic acid if its pKa value is 4.74. How is degree of dissociation affected when the solution contains (a) 0.01 M HCl (b) 0.1 M HCl ?
Solution.


Question 54.
The ionization constant of dimethylamine is 5.4 × 10-4. Calculate the degree of ionization of its 0.02 M solution. What percentage of dimethylamine is ionized if the solution is also 0.01 M NaOH ?
Solution.

Question 55.
Calculate the hydrogen ion concentration in the following biological fluids whose pH values are as follows :
(a) Human muscles fluid 6.83
(b) Human stomach fluid 1.2.
(c) Human blood 7.38
(d) Human saliva 6.4
Solution.

Question 56.
The pH of milk, black coffee, tomato juice, lemon juice and egg white are 6.8, 5.0, 4.2, 2.2 and 7.8 respectively. Calculate corresponding hydrogen ion concentration in each case.
Solution.

Question 57.
0.561 g KOH is dissolved in water to give 200 mL of solution at 298K. Calculate the concentration of potassium, hydrogen and hydroxyl ions. What is the pH of the solution ?
Solution.

Question 58.
The solubility of Sr(OH)2 at 298 K is 19.23 g/L of solution. Calculate the concentrations of strontium and hydroxyl ions and the pH of the solution.
Solution.

Question 59.
The ionisation constant of propionic acid is 1.32 × 10-5. Calculate the degree of ionisation of acid in its 0.05 M solution and also its pH. What will be its degree of ionisation if the solution is 0.01 M in HCl also ?
Solution.

Question 60.
The pH of 0.1M solution of cyanic acid (HCNO) is 2.34. Calculate ionisation constant of the acid and also its degree of dissociation in the solution.
Solution.

Question 61.
The ionisation constant of nitrous acid is 4.5 × 10-4. Calculate the pH value of 0.04 M NaNO2 solution and also its degree of hydrolysis.
Solution.

Question 62.
A 0.02 M solution of pyridinium hydrochloride has pH = 3.44. Calculate the ionisation constant of pyridine.
Solution.

Question 63.
Predict if the solutions of the following salts are neutral, acidic or basic :
NaCl, KBr, NaCN, NH4NO3, NaNO2, and KF
Solution.

Question 64.
The ionisation constant of chloroacetic acid is 1.35 × 10-3. What will be the pH of 0.1 M acid solution and of its 0.1 M sodium salt solution ?
Solution.

Question 65.
The ionic product of water at 310 K is 2.7 × 10-14. What is the pH value of neutral water at this temperature ?
Solution.

Question 66.
Calculate the pH of the resultant mixtures:
(a) 10 mL. of 0.2 M Ca(OH)2 + 25 mL of 0.1 M HCl
(b) 10 mL of 0.01 M H2SO4 + 10 mL of 0.01 M Ca(OH)2
(c) 10 mL of 0.1 M H2SO4 + 10 mL of 0.1 M KOH.
Solution.


Question 67.
Determine the solubilites of silver chromate, barium chromate and ferric hydroxide at 289 K from their solubility product constants. Determine also the molarities of the individual ions.

Solution.


Question 68.
The solubility product constants of Ag2CrO4 and AgBr are 1.1 × 10-12 and 5.0 × 10-13 respectively. Calculate the ratio of the molarities of their saturated solutions.
Solution.

Question 69.
Equal volumes of 0.002 M solutions of sodium iodate and copper chlorate are mixed together. Will it lead to the precipitation of copper iodate ? (For copper iodate Ksp = 7.4 × 10-8).
Solution.

Question 70.
The ionization constant of benzoic acid is 6.46 × 10-5 and Ksp for silver benzoate is 2.5 × 10-13. How many times is silver benzoate more soluble in a buffer of pH 3.19 compared to its solubility in pure water ?
Solution.

Question 71.
What is the maximum concentration of equimolar solutions of ferrous sulphate and sodium sulphide so that when mixed in equal volumes, there is no precipitation of iron sulphide ? (For iron sulphide, Ksp = 6.3 × 10-18).
Solution.

Question 72.
What is the minimum volume of water required to dissolve 1 g of calcium sulphate at 298 K. For calcium sulphate Ksp = 9.1 × 10-6?
Solution.

Question 73.
The concentration of sulphide ion in 0.1 M HCl solution saturated with hydrogen sulphide is 1.0 × 10-19 M. If 10 mL of this solution is added to 5 mL of 0.04 M solution of FeSO4, MnCl2, ZnCl2 and CaCl2, in which solutions precipitation will take place ?
Given Ksp for
FeS = 6.3 × 10-18,
MnS = 2.5 × 10-13,
ZnS = 1.6 × 10-24 and
CdS = 8.0 × 10-27.
Solution.