CLASS 12TH CHAPTER -11 Alcohols, Phenols and Ehers |NCERT CHEMISTRY SOLUTIONS | EDUGROWN
NCERT Solutions for Class 12 Chemistry : The NCERT solutions provided here will enhance the concepts of the students, as well as suggest alternative methods to solve particular problems to the teachers. The target is to direct individuals towards problem solving strategies, rather than solving problems in one prescribed format. The links below provide the detailed solutions for all the Class 12 Chemistry problems.
Chemistry is much more than the language of Science. We have made sure that our solutions reflect the same. We aim to aid the students with answering the questions correctly, using logical approach and methodology. The NCERT Solutions provide ample material to enable students to form a good base with the fundamentals of the subject.
NCERT Solutions for Class 12 Chemistry Chapter :11 Alcohols, Phenols and Ehers
INTEXT Questions
Question 1.
Classify the following as primary, secondary and tertiary alcohols :
Solution:
Primary alcohols : (i), (ii) and (iii)
Secondary alcohols : (iv) and (v)
Tertiary alcohols : (vi)
Question 2.
Identify allylic alcohols in the above examples.
Solution:
Allvlir alcohols : (ii) and (vi).
Question 3.
Name the following compounds according to IUPAC system.

Solution:
- 3-Chloromethyl-2-isopropylpentan-l-ol
- 2, 5-Dimethylhexane-1,3-diol
- 3-Bromocyclohexanol
- Hex-l-en-3-ol
- 2-Bromo-3-methylbut-2-en-1 -ol
Question 4.
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal.
Solution:
Question 5.
Write structures of the products of the following reactions :
Solution:

Question 6.
Give structures of the products you would expect when each of the following alcohol reacts with (a) HCl – ZnCl2 (b) HBr and (c) SOCl2.
- Butan-1-ol
- 2-Methylbutan-2-ol
Solution:
Question 7.
Predict the major product of acid catalysed dehydration of
- 1-methylcyclohexanol and
- butan-1-ol
Solution:
Question 8.
Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Solution:
The attachment of the -NO2 group to the phenol molecule at 0- and p-positions decreases the electron density on oxygen atom. This causes the oxygen atom to pull the bond pair of electrons of the O – H bond towards itself thereby facilitating the release of H as H+.
The resonance structures of the phenoxide ions are :

Question 9.
Write the equations involved in the following reactions :
- Reimer-Tiemann reaction
- Kolbe’s reaction
Solution:
Question 10.
Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Solution:
Question 11.
Which of the following is an appropriate set of reactants for the preparation of 1 -methoxy-4- nitrobenzene and why?
Solution:
Question 12.
Predict the products of the following reactions :
Solution:
NCERT Exercises
Question 1.
Write IUPAC names of the following compounds :

Solution:
- 2,2,4-Trimethylpentan-3-ol
- 5-Ethylheptan-2,4-diol
- Butane-2,3-diol
- Propane-1,2,3-triol
- 2-Methylphenol
- 4-Methylphenol
- 2,5-Dimethylphenol
- 2,6-Dimethylphenol
- l-Methoxy-2-methylpropane
- Ethoxybenzene
- 1-Phenoxyheptane
- 2-Ethoxybutane
Question 2.
Write structures of the compounds whose IUPAC names are as follows :
- 2-Methylbutan-2-ol
- 1-Phenylpropan-2-ol
- 3,5-Dimethylhexane-1,3,5-triol
- 2,3-Diethylphenol
- 1-Ethoxypropane
- 2-Ethoxy-3-methylpentane
- Cyclohexylmethanol
- 3-Cydohexylpentan-3-ol
- Cyclopent-3-en-1 -ol
- 3-Chloromethylpentan-1-ol
Solution:

Question 3.
- Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
- Classify the isomers of alcohols in question 3 (i) as primary, secondary and tertiary alcohols.
Solution:
The isomeric alcohols with molecular formula C5H12O are :

Question 4.
Explain why propanol has higher boiling point than that of the hydrocarbon, butane.
Solution:
The boiling point of any compound depends on the strength of inter-molecular forces. Stronger is the inter-molecular attraction, higher is the boiling point.
In butane, the molecules interact with each other through weak van der Waals forces. These weak forces can be easily overcome by supplying small amount of heat energy. Thus, they have low boiling point.
In propanol, the molecules are held together by strong hydrogen bonding. These attractive forces operating between molecules are more difficult to break and therefore higher amount of heat needs to be supplied, therefore, the higher boiling point.
Question 5.
Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Solution:
Organic compounds are soluble in water if they are able to form hydrogen bonds with it. Alcohols are able to establish this interaction by the virtue of their OH group and are therefore soluble in water. On the other hand, other hydrocarbons of comparable mass do not dissolve in water since they cannot form hydrogen bonds.
Question 6.
What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Solution:
Hydroboration-oxidation is a method of preparation of alcohols from alkenes. The main advantage of this method is the high yield of alcohol obtained. During hydroboration, diborane (BH3)2 is made to react with an alkene to form an addition product. This product is then treated with hydrogen peroxide in the presence of sodium hydroxide to give alcohol.
For example,
Question 7.
Give the structure and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Solution:
Question 8.
While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Solution:
During steam distillation, it is the lower boiling compound which distills out first. Between ortho- and para-nitrophenol it is the ortho-isomer which will be steam volatile since it has a lower boiling point.
The difference in boiling point between the two isomers can be understood based on the structural difference. In ortho-isomer intra¬molecular hydrogen bonding takes place while in the pttra-isomer, inter-molecular hydrogen bonding takes place.
As a result of the strong forces operating between the molecules of p-isomer, the boiling point is higher and it is not steam volatile.
Question 9.
Give the equations of reactions for the preparation of phenol from cumene.
Solution:
Question 10.
Write chemical reaction for the preparation of phenol from chlorobenzene.
Solution:
Question 11.
Write the mechanism of hydration of ethene to yield ethanol.
Solution:
The acid catalysed hydration of ethene may be represented as :

Question 12.
You are given benzene, cone. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
Solution:
Using the given reagents, phenol may be prepared as :
Question 13.
Show how will you synthesise :
- 1 -Phenylethanol from a suitable alkene.
- Cyclohexylmethanol using an alkyl halide by an SN2 reaction.
- Pentan-1-ol using a suitable alkyl halide.
Solution:

Question 14.
Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Solution:

The resonance stabilization provided by the contributing structures (I)-(V) more than compensates for the bond breakage energy of O – H bond and thus causes phenol to be acidic in nature.
No such resonance structures are possible for ethoxide ion and therefore the conversion of ethanol to ethoxide is not favoured under normal conditions. Therefore, ethanol is less acidic than phenol.
Question 15.
Explain why is ortho nitrophenol more acidic than methoxyphenol?
Solution:

Question 16.
Explain how does the -OH group attached to a carbon of benzene ring activates it towards electrophilic substitution.
Solution:
In an electrophilic substitution reaction, an electron deficient species attacks the benzene ring which is e– rich.
When an -OH group is attached to the benzene ring, by the virtue of its electron releasing nature increases the e– density of the ring and thus activates it, i.e., makes it a welcome site for electrophiles.
The increase in electron density can be visualised as :
From structures (I) – (V), we find that the attachment of hydroxyl group to benzene has increased the electron density (-ve charge) on the ring carbon atoms (especially C-2, C-4 and C-6). It is therefore said to have activated the ring towards electrophiles which are attracted to the increased electron density.
Question 17.
Give equations of the following reactions :
- Oxidation of propan-1 -ol with alkaline KMnO4 solution.
- Bromine in CS2 with phenol.
- Dilute HNO3 with phenol.
- Treating phenol with chloroform in presence of aqueous NaOH.
Solution:

Question 18.
Explain the following with an example.
(i) Kolbe’s reaction
(ii) Reimer-Tiemann reaction
(iii) Williamson ether synthesis
(iv) Unsymmetrical ether.
Solution:
(i) Kolbe’s reaction :
The fact that phenoxide ion is even more reactive than phenol towards incoming electrophiles is made use of in this reaction. Sodium phenoxide is reacted with CO2 followed by acid treatment to yield o-hydroxybenzoic acid as the major product.
(ii) Reimer-Tiemann reaction :
Treatment of phenol with chloroform in the presence of aqueous alkali introduces a CHO group at the ortho position. Acidification yields salicylaldehyde.
(iii) Williamson synthesis :
In this method, an alkyl halides is reacted with sodium alkoxide.
R-X + R’ – ONa → R-O-R’ + NaX
The reaction involves SN2 attack of an alkoxide ion on 1° RX.
(iv) Unsymmetrical ethers :
Unsymmetrical ethers are organic compounds where the ethereal oxygen atom is attached to two different alkyl or aryl groups, e.g.,
C2H5 – O – CH3, C6H5O – C2H5, etc.
Question 19.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Solution:
Question 20.
How are the following conversions carried out?
- Propene → Propan-2-ol
- Benzyl chloride → Benzyl alcohol
- Ethyl magnesium chloride → Propan-1-ol
- Methyl magnesium bromide → 2-Methylpropan-2-ol.
Solution:

Question 21.
Name the reagents used in the following reactions :
- Oxidation of primary alcohol to carboxylic acid.
- Oxidation of primary alcohol to aldehyde.
- Bromination of phenol to 2,4,6-tribromophenol.
- Benzyl alcohol to benzoic acid.
- Dehydration of propan-2-ol to propene.
- Butan-2-one to butan-2-ol.
Solution:
- Alkaline KMnO4
- Pyridinium chlorochromate in chloromethane (CH2Cl2)
- Br2/H2O
- Alkaline KMnO4
- Cone. H2SO4 or H3PO4 at 433-443 K
- H2/Ni or NaBH4 or LiAlH4
Question 22.
Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Solution:
The higher boiling point of ethanol may be attributed to the presence of intermolecular hydrogen bonding in it.
Due to such extensive bonding, more energy needs to be supplied to ethanol to break these bonds and move it into the vapour phase.
Question 23.
Give IUPAC names of the following ethers :
Solution:
- 1 -Ethoxy-2-methylpropane
- 2-Chloro-l-methoxyethane
- 4-Nitroanisole
- 1-Methoxypropane
- 1-Ethoxy-4,4-dimethylcyclohexane
- Ethoxybenzene
Question 24.
Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis :
- 1-Propoxypropane
- Ethoxybenzene
- 2-Methyl-2-methoxypropane
- 1-Methoxyethane
Solution:

Question 25.
Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Solution:
The main limitation of Williamson’s ether synthesis lies in its unemployability for preparation of unsymmetrical ethers where the compound contains secondary or tertiary alkyl groups.
e.g., reaction between tert-butyl bromide and sodium methoxide yields an alkene.
This is because the competing elimination reaction predominates over SN2 and alkene is formed.
Question 26.
How is 1-propoxypropane synthesised from propan-1 -ol? Write mechanism of this reaction.
Solution:
Question 27.
Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Solution:
Consider the reaction between propan- 2-ol molecules in the presence of acid.
If an ether is to be formed, another alcohol molecule must carry out a nucleophilic attack on the carbocation as
However, this does not happen because of
(a) the steric hindrance around the carbocation, and
(b) bulky size of the nucleophile which would further cause crowding.
As a result, the carbocation prefers to lose a proton and forms an alkene.
For the same reason 3° alcohols in the presence of acid do not form ethers since 3° alcohols are even more sterically hindered than 2° alcohols.
Question 28.
Write the equation of the reaction of hydrogen iodide with :
- 1-propoxypropane
- methoxybenzene; and
- benzyl ethyl ether.
Solution:
Question 29.
Explain the fact that in aryl alkyl ethers (i) the alkoxy group activates the benzene ring towards electrophilic substitution and (ii) it directs the incoming substituents to ortho and para positions in benzene ring.
Solution:
Consider the following resonance structures of aryl alkyl ethers :
(i) From the above structures we find that the presence of the OR group has increased the electron density on the benzene ring and therefore the ring is said to have been activated towards incoming electrophiles.
(ii) From structures (II), (III) and (IV) we find that and the electron density has increased on C-2, C-4 and C-6, i.e., at the ortho and para positions. As a result the electrophile (E ) attaches itself to these e- rich sites and the -OR group is said to have directed the E® to ortho and para positions.
Question 30.
Write the mechanism of the reaction of Hl with methoxymethane.
Solution:
The reaction between methoxymethane and Hl is :
Question 31.
Write equations of the following reactions :
- Friedel-Crafts reaction – alkylation of anisole.
- Nitration of anisole.
- Bromination of anisole in ethanoic acid medium.
- Friedel-Craft’s acetylation of anisole.
Solution:

Question 32.
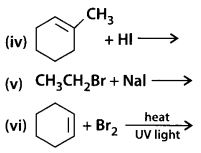
Show how would you synthesise the following alcohols from appropriate alkenes.
Solution:

Question 33.
When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place :
Give a mechanism for this reaction.
[Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.]
Solution: